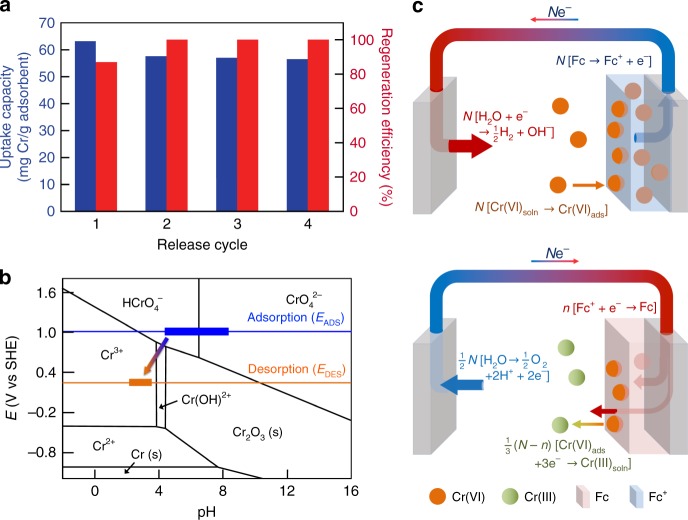
Fig. 2.
Investigation of the reversibility of the electrochemically mediated capture-and-release process. a Recyclability of the electrode over a number of discharge cycles (+0.8 V adsorption, 0 V discharge), as given by the normalized mass released (in blue), and the regeneration efficiency (%) (in red), the latter denoting the relative amount of Cr recovered relative to that adsorbed in each cycle. b The E-pH diagram for chromium speciation predominance, constructed with commercial thermochemical software (FactSage) at 25 °C for 1 mM total chromium concentration in the liquid phase. The adsorption and desorption potentials are noted, with the range of solution conditions marked both for adsorption (in blue) and desorption (in orange). c The Faradaic reactions occurring at the surface of the electrode pair are shown during both adsorption and release. During adsorption, hexavalent chromate is captured by the anode through selective binding, whereas during release, reduction of ferrocenium to ferrocene and of Cr(VI) to Cr(III) occurs