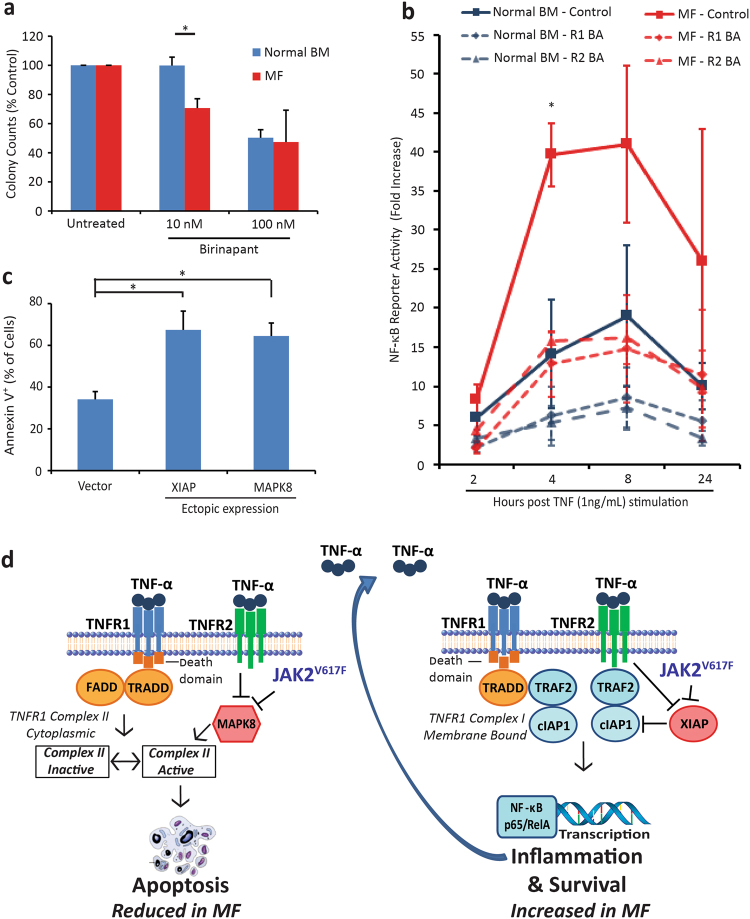
Fig. 5.
JAK2V617F and TNFR2 cooperate to increase NF-κB signaling and reduce apoptosis in MF cells. a Human CD34+ cells isolated from MF patient (n = 3) or normal BM (n = 3) were treated with birinapant at 10 or 100 nM for 72 h in liquid culture and then plated in clonogenic assays with continued treatment. Colony inhibition was significantly different for normal vs. MF samples at 10 nM, while 100 nM birinapant inhibited both. b Human CD34+ cells isolated from MF patient (n = 3) or normal BM (n = 3) were infected with an NF-κB luciferase reporter construct 72 h prior to evaluation. Cells were treated with TNFR1 or TNFR2 BA (10 μg/mL) prior to stimulation with TNF (1 ng/mL). The fold increase in reporter activity was significantly higher in MF cells at 4 h post stimulation and over the complete time course (P = 0.03). Both TNFR1 and TNFR2 BAs reduced TNF stimulated NF-κB activity in MF and normal BM cells. c Annexin V was measured in MF CD34+ cells (n = 4) 72 h after infection with XIAP, MAPK8 or vector control expression constructs. Ectopic expression of XIAP or MAPK8 significantly increased Annexin V staining relative to vector control. d Downregulation of MAPK8 by JAK2V617F and TNFR2 inhibits apoptotic signaling through TNFR1 by preventing MAPK8 from promoting the active form of TNFR1 Complex II. Downregulation of XIAP by JAK2V617F and TNFR2 is associated with increased cIAP protein levels. Either TNFR1 (with TRADD) or TNFR2 can form a signaling complex through association with TRAF2 and cIAP to activate NF-κB transcription of pro-survival and inflammation-associated genes. These combined effects favor survival of JAK2V617F cells. *P < 0.05, **P < 0.005