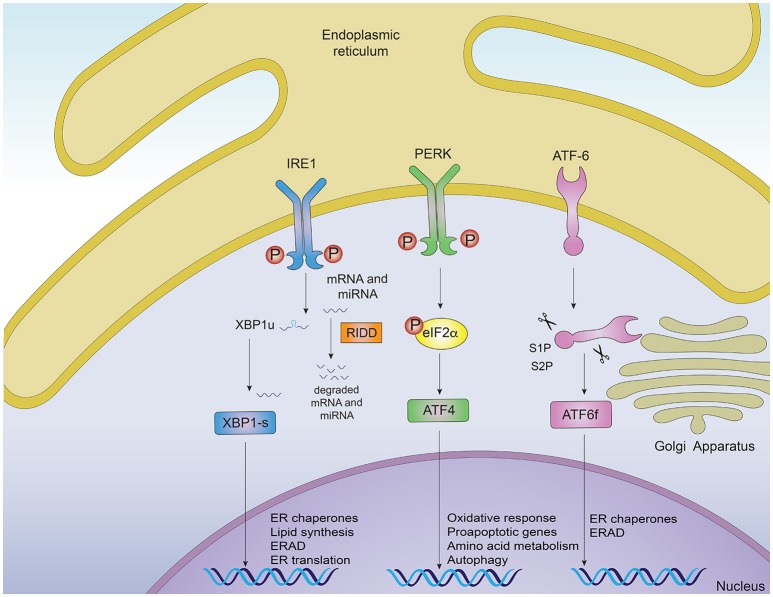
Figure 1.
Signaling pathways of the unfolded protein response. Noxious stimuli in cells may induce endoplasmic reticulum (ER) stress and trigger an adaptive response known as the unfolded protein response (UPR), which is controlled by three main ER-resident sensors: IRE1α, PERK and ATF6. Upon ER stress, IRE1α autophosphorylates, leading to the activation of its RNase domain and the processing of the mRNA encoding for XBP1s, a transcriptional factor that upregulates genes involved in protein folding and quality control, in addition to regulating ER/Golgi biogenesis and ER-mediated degradation (ERAD). Additionally, IRE1α RNase also degrades a subset of specific RNAs and microRNAs, a process termed Regulated IRE1α-Dependent Decay (RIDD). The second ER sensor, PERK, phosphorylates the translation of the eukaryotic initiation factor eIF2α, decreasing the synthesis of proteins and the overload of misfolded proteins at the ER. PERK phosphorylation also leads to the specific translation of ATF4, a transcription factor that promotes the expression of genes related to amino acid metabolism, anti-oxidant response, autophagy and apoptosis. The third UPR sensor, ATF6, is a type II ER transmembrane protein that encodes a bZIP transcriptional factor in its cytosolic domain. Following ER stress, ATF6 translocates to the Golgi apparatus where it is processed, releasing a transcription factor which directs the expression of genes encoding ER chaperones, ERAD components and molecules involved in lipid biogenesis.