Figure 1.
G6Pase-β Is Expressed in Astrocytes from Rodents and Humans
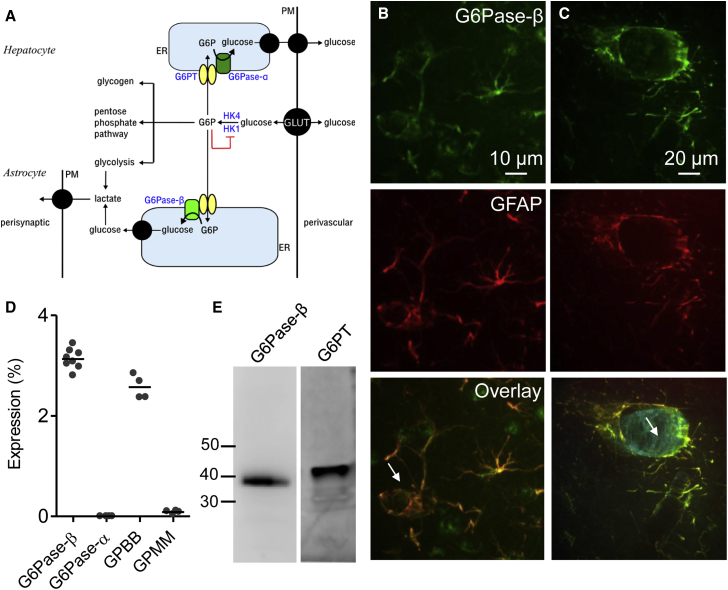
(A) Glucose transported into cells by glucose transporters (GLUT) is phosphorylated to G6P by HK. Glucose-exporting cells, like hepatocytes, use a G6P transporter (G6PT) to transport G6P into the ER, where it is dephosphorylated by G6Pase-α and then exported from the cell, possibly at ER-PM contact sites (top right). G6PT and G6Pase-α are viewed as adaptations that allow efficient glucose export. Astrocytes are proposed to provide neurons with a source of energy and neurotransmitter precursors by importing glucose at their perivascular endfeet, glycolytically metabolizing it, and then exporting neurotransmitter precursors and perhaps lactate at perisynaptic processes [17]. Although the importance of the lactate shuttle has been questioned [10, 11, 12], it is clear that astrocytes provide metabolic support to neurons. Our results suggest that G6PT and G6Pase-β allow the ER of astrocytes to serve as an intracellular highway moving glucose from perivascular endfeet to perisynaptic processes.
(B and C) Confocal z stacks of rat brain cortical slices immunostained for G6Pase-β and GFAP showing their colocalization. Arrows in the overlays indicate the lumen of blood vessels surrounded by astrocytes. The overlay in (C) is additionally stained with isolectin B4 to identify capillaries (blue).
(D) qPCR showing expression levels of mRNA (relative to GAPDH) of the indicated enzymes in human astrocytes: GPBB and GPMM are two isoforms of glycogen phosphorylase. Results show each independent determination (n = 4–8 isolates, derived from at least 4 different cultures) and the mean.
(E) Immunoblots (30 μg protein/lane), typical of 3 similar blots from independent treatments, show expression of G6Pase-β and G6PT in human astrocytes.