Figure 2.
The ER of Human Astrocytes Sequesters Glucose by Importing G6P
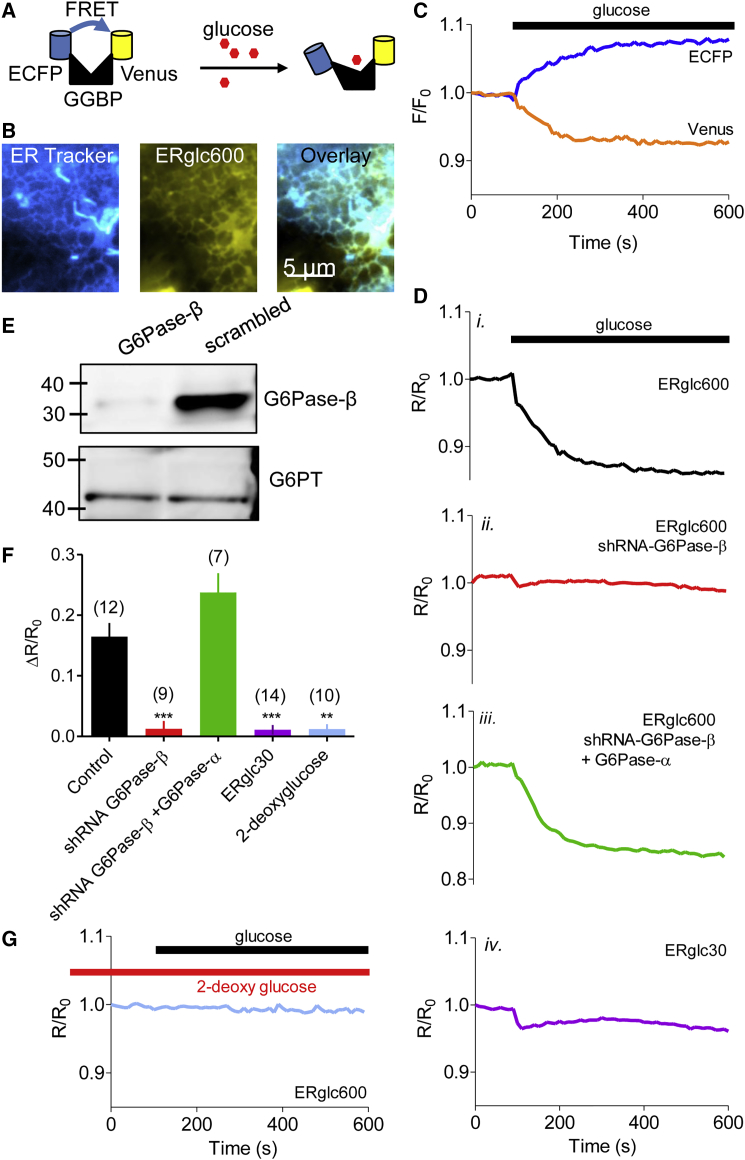
(A) The ER glucose-sensors comprise a glucose-binding protein (GGBP) tethered to enhanced cyan fluorescent protein (ECFP) and Venus, such that glucose binding separates the chromophores causing a decrease in FRET efficiency.
(B) Total internal reflection fluorescence (TIRF) image of astrocyte showing colocalization of ER-Tracker Red (shown in blue) with ERglc600 (yellow).
(C) Typical trace from a single astrocyte expressing ERglc600 and exposed to glucose (5 mM, bar) showing reciprocal changes in the fluorescence of Venus and ECFP (F/F0, where F0 is the fluorescence recorded before adding glucose).
(D) FRET ratios (R/Ro, Venus/ECFP) were recorded using ERglc600 (i, ii, iii) or ERglc30 (iv) after adding glucose (5 mM) to normal astrocytes (i, iv), after shRNA-mediated knockdown of G6Pase-β (ii) alone or with expression of G6Pase-α (iii).
(E) Western blots (WBs) (30 μg protein/lane), typical of 3 independent transfections, show effects of G6Pase-β shRNA and scrambled shRNA on expression of G6Pase-β and G6PT. Positions of Mr markers (kDa) are shown.
(F) Summary results (mean ± SEM from (n) independent cells; n shown above bars) show R/Ro determined 250 s after addition of glucose or 2-deoxyglucose. ∗∗∗p < 0.001, ∗∗p < 0.01, Kruskal-Wallis with Dunn’s multiple comparisons test, relative to control.
(G) Analysis of astrocytes expressing ERglc600 and pretreated with 2-deoxglucose (5 mM, 30 min) to inhibit HK before addition of glucose (5 mM).