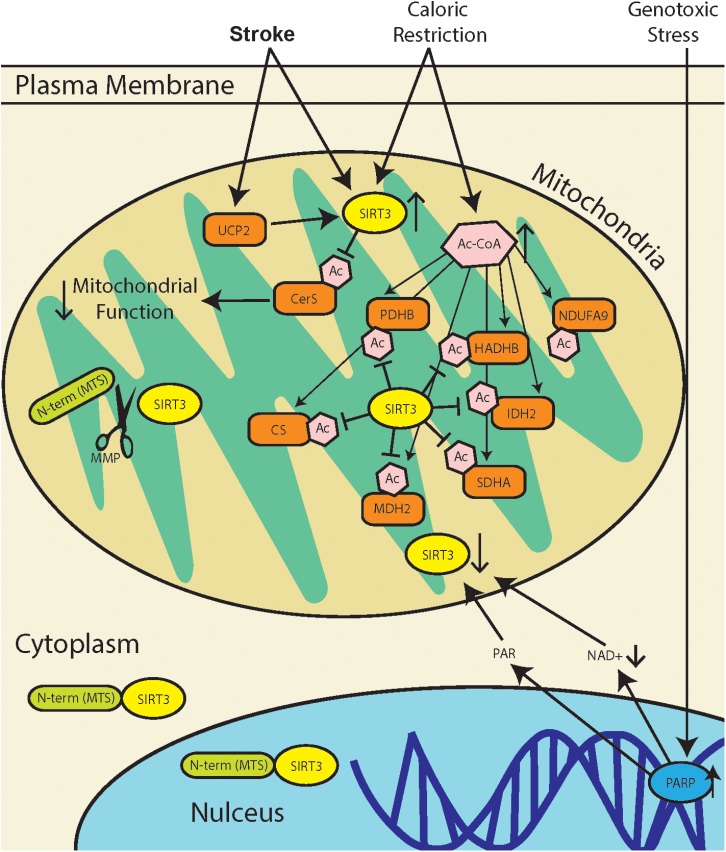
FIGURE 1.
SIRT3 regulation during stress response. Stroke results in a combination of genotoxic, oxidative, and metabolic stresses, all of which have different effects on SIRT3 activity. Metabolic stress due to caloric restriction result in an increase in acetyl-CoA (Ac-CoA) which can acetylate proteins through non-enzymatic mechanisms (Weinert et al., 2015). SIRT3 can then repair acetylated mitochondrial proteins including pyruvate dehydrogenase (PDHB), citrate synthase (CS), acetyl-CoA acyltransferase (HADHB), isocitrate dehydrogenase 2 (IDH2), NADH dehydrogenase alpha subcomplex subunit 9 (NDUFA9), malate dehydrogenase 2 (MDH2), and succinate dehydrogenase complex subunit A (SDHA). Genotoxic stress, which occurs after stroke, leads to poly(ADP-ribose) polymerase (PARP) hyperactivation. This leads to a decrease in [NAD+], as well as accumulation of poly(ADP-ribose) polymer (PAR), and this may lead to downregulation of SIRT3. Mitochondria dysfunction in stroke can increase expression of mitochondrial uncoupling protein 2 (UCP2), which decreases ATP production and increases mitochondrial NAD+/NADH ratio, thereby increasing SIRT3 activity. Recent reports of SIRT3 activity in stroke demonstrate that SIRT3 deacetylates and activates ceramide synthases (CerS) which results in mitochondrial dysfunction.