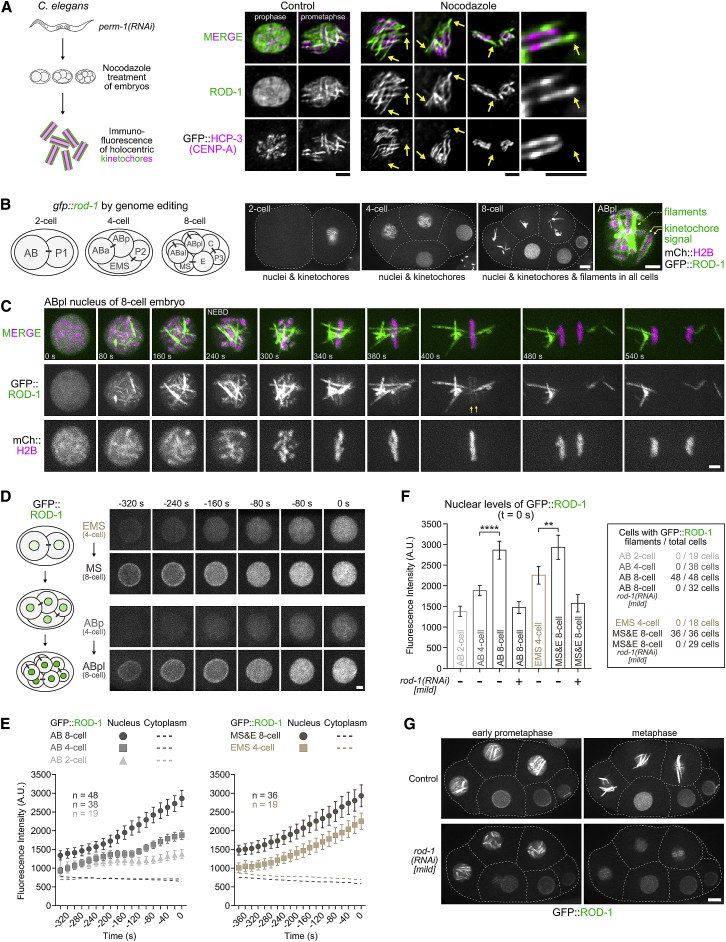
Figure 4.
C. elegans ROD-1 Is Capable of Self-Assembly into Micrometer-Scale Filaments In Vivo
(A) (Left) Schematic of experimental protocol to visualize kinetochore expansion in C. elegans early embryos. RNAi-mediated depletion of PERM-1 permeabilizes the eggshell of embryos, which are subsequently isolated from hermaphrodite adults, treated with nocodazole, and immunostained for ROD-1 and the centromere marker GFP::HCP-3CENP-A. (Right) Immunofluorescence images of mitotic embryonic cells with and without nocodazole treatment. Arrows point at filamentous kinetochore expansions containing ROD-1 that form in the absence of microtubules. Scale bars, 2 μm.
(B) (Left) Schematic of the C. elegans early embryo at the two-, four-, and eight-cell stages. Names of individual cells are indicated. Bars connecting cells indicate they originated from the same mother cell. (Right) Selected images from a time-lapse sequence of an early embryo expressing endogenous ROD-1 tagged with GFP. GFP::ROD-1 is enriched in nuclei and localizes transiently to holocentric kinetochores in mitosis. In addition, GFP::ROD-1 starts to form filaments during mitosis at the eight-cell stage, but not earlier (see also Video S1). Dashed lines mark cell boundaries. Scale bars, 5 μm; blow-up, 2 μm.
(C) Selected images from a time-lapse sequence documenting the formation of GFP::ROD-1 filaments during mitosis at the eight-cell stage (see also Video S2). mCherry::histone H2B labels chromosomes. Filaments, typically several micrometers in length, form in the nucleus before NEBD and segregate to daughter cells by clustering at spindle poles. Kinetochore-localized GFP::ROD-1 is also visible (arrows). Time point 0 refers to the last frame before the appearance of GFP::ROD-1 on filaments and kinetochores. Scale bar, 2 μm.
(D) (Left) Schematic highlighting the increase in nuclear GFP::ROD-1 levels during early embryonic development. (Right) Selected images of nuclei from a time-lapse sequence of a developing embryo expressing GFP::ROD-1 that was followed from the two-cell stage to the eight-cell stage. (Top) Images show the EMS cell in the four-cell embryo, which gives rise to the MS cell in the eight-cell embryo. (Bottom) Likewise, the ABp cell gives rise to the ABpl cell. In both instances, nuclear GFP::ROD-1 levels increase gradually during the cell cycle and are significantly higher in daughter cells. Time point 0 denotes the last frame before GFP::ROD-1 appears on kinetochores (EMS and ABp) or filaments (MS and ABpl). Similar results were obtained for nuclei of the P lineage (not shown). Scale bar, 2 μm.
(E) Quantification of average GFP::ROD-1 signal in nuclei and the cytoplasm in developing embryos as shown in (D). Average fluorescence intensity was determined in images acquired every 20 s, averaged for the indicated number n of cells from at least 8 embryos, and plotted against time. Time point 0 denotes the last frame before the appearance of GFP::ROD-1 on filaments and/or kinetochores. Values are shown as mean ± 95% confidence interval for nuclear signal and as the mean for cytoplasmic signal.
(F) (Left) Quantification of nuclear GFP::ROD-1 levels in cells at different developmental stages. Measurements correspond to the last frame before GFP::ROD-1 appears on filaments and/or kinetochores (time point 0 s), showing a significant increase of nuclear signal at the eight-cell stage. Mild rod-1(RNAi) was used to reduce GFP::ROD-1 levels. Values are shown as mean ± 95% confidence interval. Statistical significance was determined by one-way ANOVA followed by Bonferroni's multiple comparison test. ∗∗∗∗p < 0.0001; ∗∗p < 0.01. (Right) Table showing that filament formation commences strictly at the eight-cell stage and can be completely suppressed by mildly reducing GFP::ROD-1 levels.
(G) Selected images from eight-cell embryos whose AB lineage cells are going through mitosis. Mild depletion of GFP::ROD-1 slightly lowers enrichment in nuclei, which suppresses filament formation but does not prevent GFP::ROD-1 localization to kinetochores. Scale bar, 5 μm.
See also Figure S4.