Abstract
Introduction
Despite the high incidence of posttransplant diabetes mellitus (PTDM) among high-risk recipients, no studies have investigated its prevention by immunosuppression optimization.
Methods
We conducted an open-label, multicenter, randomized trial testing whether a tacrolimus-based immunosuppression and rapid steroid withdrawal (SW) within 1 week (Tac-SW) or cyclosporine A (CsA) with steroid minimization (SM) (CsA-SM), decreased the incidence of PTDM compared with tacrolimus with SM (Tac-SM). All arms received basiliximab and mycophenolate mofetil. High risk was defined by age >60 or >45 years plus metabolic criteria based on body mass index, triglycerides, and high-density lipoprotein–cholesterol levels. The primary endpoint was the incidence of PTDM after 12 months.
Results
The study comprised 128 de novo renal transplant recipients without pretransplant diabetes (Tac-SW: 44, Tac-SM: 42, CsA-SM: 42). The 1-year incidence of PTDM in each arm was 37.8% for Tac-SW, 25.7% for Tac-SM, and 9.7% for CsA-SM (relative risk [RR] Tac-SW vs. CsA-SM 3.9 [1.2–12.4; P = 0.01]; RR Tac-SM vs. CsA-SM 2.7 [0.8–8.9; P = 0.1]). Antidiabetic therapy was required less commonly in the CsA-SM arm (P = 0.06); however, acute rejection rate was higher in CsA-SM arm (Tac-SW 11.4%, Tac-SM 4.8%, and CsA-SM 21.4% of patients; cumulative incidence P = 0.04). Graft and patient survival, and graft function were similar among arms.
Conclusion
In high-risk patients, tacrolimus-based immunosuppression with SM provides the best balance between PTDM and acute rejection incidence.
Keywords: cyclosporin A, posttransplant diabetes, posttransplant hyperglycemia, renal transplantation, steroid withdrawal, tacrolimus
See Commentary on Page 1249
PTDM is a frequent and severe complication of renal transplantation (RT).1, 2, 3 With current immunosuppression regimens, the incidence of PTDM at 1 and 3 years is 20% and 30%, respectively.4 Importantly, PTDM is an established risk factor for cardiovascular disease and mortality, and accordingly, is associated with increased health care costs.5, 6, 7, 8 However, few studies have been designed to prevent this condition.9
RT recipients with risk factors for type 2 diabetes most commonly develop PTDM after exposure to immunosuppressants.2, 10, 11 These risk factors include age older than 45 years at the time of transplantation, overweight, obesity, and hypertriglyceridemia, a known marker of insulin resistance.12, 13, 14, 15, 16, 17 For instance, a retrospective study observed that each 50-mg/dl increment in serum levels of triglycerides determined a 30% increase in the risk for PTDM.16 Moreover, the higher the number of preexisting risk factors, the higher the frequency of PTDM.11, 15 Although the immunosuppressive regimen explains 74% of the risk of PTDM,18, 19 the best immunosuppressive approach in patients at risk for developing PTDM is not yet defined.
Early SW posttransplantation is associated with a reduction in the RR of PTDM.20, 21 However, 2 recent studies in patients treated with tacrolimus yielded conflicting results. Although SW at day 8 reduced PTDM at 1 year from 39% in maintenance steroid patients to 24%,22 others observed no differences after 5 years between patients with early SW and chronic maintenance steroids.23 Thus, the role of early SW in patients at risk for PTDM is not yet established.
Tacrolimus reduces insulin secretion both in vitro and in vivo more potently than CsA.24, 25 In the DIRECT trial, the only study comparing CsA and tacrolimus with PTDM as the primary endpoint, CsA was superior to tacrolimus.25 Furthermore, the effects of tacrolimus are exacerbated in patients with type 2 diabetes susceptibility, a phenomenon not observed with CsA.16, 26, 27 This interaction has also been demonstrated in Zucker rats, in which tacrolimus induces higher rates of diabetes in obese and insulin-resistant animals than CsA, but not in nonobese animals.28 Conversion from tacrolimus to CsA in both insulin-resistant rodents and patients reverses diabetes in 35% to 50% of cases,29, 30, 31 improving beta-cell secretion, proliferation, and insulin gene expression in vivo.29 We, therefore, reasoned that CsA may be an alternative to tacrolimus to decrease early beta-cell damage in patients at high risk for PTDM.
The primary objective of this open, randomized controlled trial was to investigate the incidence of PTDM in high-risk patients under a tacrolimus-based immunosuppression and rapid SW within 1 week, or CsA with SM, compared with tacrolimus and SM.
Methods
Study Design
This study was an investigator-driven, open-label, multicenter, prospective, randomized phase IV clinical trial of 12-month duration in which de novo RT patients with a low immunological risk and high risk for PTDM were randomized 1:1:1 to 3 arms: tacrolimus and rapid SW in 1 week (Tac-SW), CsA with SM (CsA-SM), and tacrolimus with SM (Tac-SM) as a control group. All groups received induction with basiliximab. All patients signed an informed consent, and the study was approved by the ethics and clinical research committee of each participating center.
Study Population
We included patients with end-stage renal disease who received a first RT in the absence of (i) pretransplant diabetes, defined as baseline blood glucose ≥126 mg/dl or treatment with hypoglycemic medication; (ii) immunological risk defined by panel-reactive antibody score <50% plus investigator's criteria; and (iii) infection by hepatitis C and/or B viruses. Additionally, at least 1 of the following “metabolic criteria” were required: (i) recipient age ≥60 years, or (ii) recipient age between 45 and 59 years plus 1 of the following criteria: (i) pretransplant triglyceride level ≥200 mg/dl, (ii) body mass index >27 kg/m2 plus triglycerides >150 mg/dl, or (iii) high-density lipoprotein cholesterol <40 mg/dl in men or 50 mg/dl in women plus triglycerides >150 mg/dl.
We excluded patients who received a graft that, in the opinion of the investigator, required a delay in the initiation of calcineurin inhibitors supported by induction with thymoglobulin. We also excluded patients with double kidney transplants or transplants of a kidney plus another organ.
Randomization, Arms, and Interventions
After signing the informed consent, patients were randomized 1:1:1 with a computerized algorithm generated in the Research Unit of Hospital Universitario de Canarias.
-
•
Tac-SW arm: tacrolimus (Prograf) 0.15 mg/kg per day p.o. in 2 separate doses to maintain trough levels of 8 to 12 ng/ml in the first month, and mycophenolate mofetil (MMF; Cell Cept) 2 g/d p.o. Methyl-prednisolone 0.5 g i.v. intraoperatively and 125 mg on day 1; prednisone 30 mg p.o. on days 2 and 3, 20 mg on day 4, 15 mg on day 5, 10 mg on day 6, 5 mg on day 7, and then discontinuation.
-
•
Tac-SM arm: tacrolimus and MMF following the same schedule as in arm 1. Intraoperative and day 1 methyl-prednisolone as in arm 1; prednisone 0.3 mg/kg per day p.o. from day 2 to 7 (never >20 mg/d), 0.2 mg/kg per day from day 8 to 14 (never >15 mg/d), 0.15 mg/kg per day from day 15 to 21 (never >10 mg/d), 0.1 mg/kg per day from day 22 to 28 (never >7.5 mg/d), and then 5 mg/d until 5 months, with subsequent gradual discontinuation over 4 weeks.
-
•
CsA-SM arm: Cyclosporine A microemulsion (Neoral) (CsA) 5 mg/kg per day p.o. to maintain C0 levels of 150–200 ng/ml the first month, and MMF and steroids following the same schedule as arm 2.
Basiliximab, 20 mg i.v. on days 0 and 4, was administered in all arms. In the tacrolimus arms, the MMF was reduced to 1 g/d from the first month, but in the CsA-SM arm, the target was a dose of 2 g/d.
In all 3 arms, tacrolimus and CsA levels were reduced from the first month according to usual clinical practice until 1 year, when target levels of tacrolimus were established at 5 to 8 ng/ml, and those of CsA at 100–150 ng/ml.
Conversion from CsA to tacrolimus was contemplated in CsA-SM patients who developed acute rejection as per the investigator’s criteria. Likewise, conversion from a calcineurin inhibitor to mammalian target of rapamycin inhibitor was done when considered necessary from clinical practice, such as in severe nephrotoxicity, neoplasia, or viral infection. Steroids were maintained or re-introduced at low doses (5 mg/d of prednisone) in the case of acute rejection, conversion to mammalian target of rapamycin inhibitor, or if it was considered clinically necessary to balance immunosuppression, at the investigator’s discretion, such as in the event of MMF dose reduction due to leukopenia or diarrhea.
Biopsy-proven acute rejection was classified according to the Banff 2007 criteria.32 Rejections were treated with 3 boluses of 500 mg/d of i.v. methyl-prednisolone. Corticoresistant rejections were treated with rabbit thymoglobulin. Acute humoral rejections were treated with 3 boluses of methyl-prednisolone and plasmapheresis, plus i.v. Igs and/or rituximab.
Universal pneumocystis jirovecii prophylaxis was given with cotrimoxazole, and cytomegalovirus prophylaxis in at-risk patients according to the protocol of each participating center.
In case of hyperglycemia in the postoperative period, rapid insulin therapy was initiated when fasting capillary blood glucose was ≥180 mg/dl. During the study, patients who developed diabetes received antidiabetic therapy based on the international guidelines available at the start of the study.33
Evaluation
In addition to routine laboratory determinations, fasting blood glucose was measured each day on days 3 through 7, as well as at months 3, 6, and 12, and HbA1c at 3 and 12 months posttransplantation. A standard oral glucose tolerance test (OGTT) of 75 g glucose was performed at 3 and 12 months, except in those patients who had already developed PTDM. Fasting insulin levels were measured at 3 and 12 months posttransplantation as described,34 and the McAuley index was calculated as an estimate of insulin resistance.35 The glomerular filtration rate was estimated by MDRD 4, and proteinuria was quantified either in 24-hour urine or as the protein/creatinine ratio in the first voided morning sample by standard methods.
Efficacy and Safety Variables
The primary endpoint was the presence of PTDM based on the American Diabetes Association criteria.36 For the purposes of the study, patients were classified based on 2 criteria: criterion 1, those in whom fasting blood glucose levels were ≥126 mg/dl, HbA1c ≥6.5%, or received hypoglycemic treatment; criterion 2, which includes all the cases classified by criterion 1 plus those in whom PTDM was diagnosed only by the OGTT (blood glucose levels at 120 minutes ≥200 mg/dl).36 Glucose intolerance was defined as blood glucose ≥140 mg/dl and <200 mg/dl 120 minutes after the OGTT.36
The major safety variables were the biopsy-proven acute rejection rate, estimated glomerular filtration rate, proteinuria, and graft and patient survival.
Statistical Analysis: Sample Size
Both intention-to-treat and per-protocol analyses were performed. We used the 1-way analysis of variance test to compare continuous variables between groups. The proportion of patients in each study arm with PTDM or glucose intolerance was compared by χ2 test and also expressed as RR with 95% confidence interval. The Kaplan-Meier and log-rank test were used to compare graft and patient survival, as well as the cumulative incidence of acute rejection.
At study design, the definition of PTDM varied considerably between studies, making meaningful comparisons of its incidence difficult. Also, few data on high-risk patients were available. With these limitations, the sample size was calculated based on previous publications in which the rate of PTDM in patients on tacrolimus with rapid SW was 8%20 versus 27% in patients on tacrolimus with maintenance steroids in a clinical trial conducted in Spain.37 For high-risk patients, a similar 2.7 proportional reduction was assumed. Thus, with a statistical power of 80% and a 2-tailed significance level (alpha) of 0.05, 64 subjects per group were required. For CsA, we expected a reduction in PTDM of at least 50%, as observed in the Symphony study.38 Therefore, we planned to include a similar number in this group. Assuming a 10% loss rate we started the study with the intention of including 210 subjects, 70 per arm.
An intermediate analysis when the first 100 patients had completed the study was planned.
Results
Patients Included
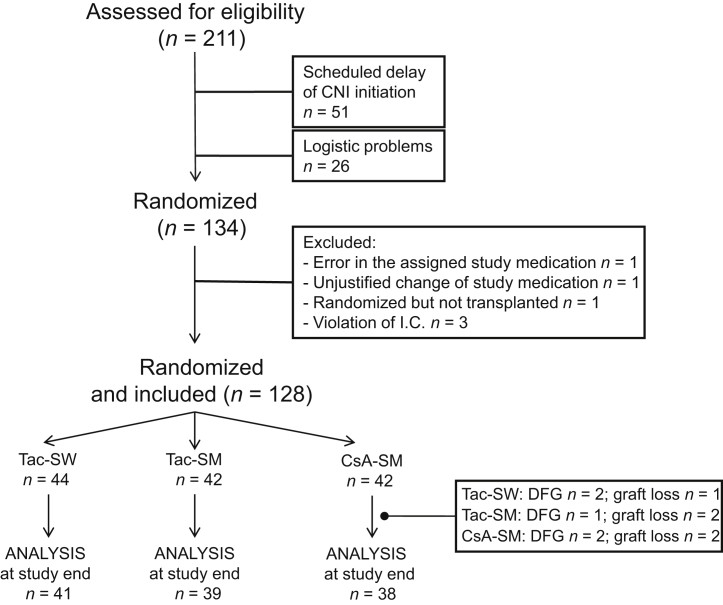
Eight transplant centers participated in the study, which began on February 23, 2010. In the intermediate analysis, a higher frequency of acute rejection was observed in the CsA arm (Tac-SW 14%, Tac-SM 3%, and CsA-SM 20%). The Safety Committee decided to stop recruitment but continue with the patients already recruited until the end of the study. Therefore, until February 5, 2014, a total of 128 patients were recruited (Figure 1).
Figure 1.
Patient disposition. CNI, calcineurin inhibitor; CsA-SM, cyclosporine A and steroid minimization; I.C., inclusion criteria; DFG: death with a functioning graft; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
Demographics of Patients and Donors
More than 90% of the recipients were white and none had a panel-reactive antibody score >25% (Table 1). All donors were deceased except 1 living donor. The arms were comparable except for a greater number of HLA mismatches in the CsA-SM versus Tac-SW arm. Although nonsignificant, a family history of diabetes was 2- to 3-fold more common in the CsA-SM arm. The rate of delayed graft function was high (52%), corresponding to the high frequency of expanded criteria donors (60%).
Table 1.
Baseline characteristics of the 3 study arms (original assigned groups)
| Variable | Tac-SW (n = 44) | Tac-SM (n = 42) | CsA-SM (n = 42) | All (n = 128) | P |
|---|---|---|---|---|---|
| Age (yr) | 61.2 ± 7.6 | 61.6 ± 7.3 | 60.2 ± 8.3 | 61 ± 7.7 | 0.7 |
| Gender (% female) | 11 (25) | 12 (28.6) | 12 (28.6) | 35 (27.3) | 0.9 |
| Race | 41W/1NA/2H | 38W/1NA/3H | 39W/1BR/1NA/1H | 118W/1BR/3NA/6H | 0.8 |
| Dialysis duration (mo) | 37.9 ± 30 | 28.6 ± 23.6 | 33 ± 26.3 | 33.2 ± 26.8 | 0.3 |
| Donor age (yr) | 62.1 ± 10.4 | 62.9 ± 8.9 | 61.6 ± 10 | 62.2 ± 9.8 | 0.8 |
| Donor creatinine (mg/dl) | 0.8 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.6 |
| Expanded criteria donor (%) | 25 (57) | 26 (62) | 26 (62) | 77 (60) | 0.9 |
| Cold ischemia time (h) | 16.8 ± 5.5 | 18.2 ± 5.4 | 17.3 ± 6.4 | 17.4 ± 5.8 | 0.6 |
| HLA-A-B-DR mismatches | 3.6 ± 1.2 | 4 ± 0.9 | 4.2 ± 1.1a | 3.9 ± 1.1 | 0.01 |
| PRA (>0 and <25%) | 3 | 0 | 1 | 4 | 0.3 |
| Delayed graft function (%) | 17/44 (38.6) | 24/42 (57.1) | 25/42 (59.5) | 66/128 (51.5) | 0.09 |
| Body mass index (kg/m2) | 26.9 ± 3.7 | 27.9 ± 3.7 | 27.9 ± 4.1 | 27.6 ± 3.8 | 0.4 |
| Family history of diabetes (%) | 3 (8) Unknown: 6 |
4 (12) Unknown: 9 |
9 (23) Unknown: 3 |
16 (14.5) Unknown: 18 |
0.2 |
| Fasting glucose (mg/dl) | 91.9 ± 13.2 | 97.7 ± 14.3 | 91.9 ± 13.9 | 93.9 ± 14 | 0.09 |
| HbA1c (%) | 5.3 ± 0.5 | 5.4 ± 0.3 | 5.3 ± 0.4 | 5.3 ± 0.4 | 0.8 |
| Triglycerides (mg/dl) | 184.3 ± 11.9 | 199.3 ± 92 | 209.7 ± 109 | 197.6 ± 105.8 | 0.5 |
| Total cholesterol (mg/dl) | 165.9 ± 48 | 162.8 ± 39.3 | 179.4 ± 42.5 | 169.4 ± 43.8 | 0.2 |
| HDL cholesterol (mg/dl) | 41 ± 14.4 | 42.5 ± 12.1 | 42.2 ± 15.3 | 41.9 ± 13.9 | 0.9 |
| LDL cholesterol (mg/dl) | 98.5 ± 36.6 | 90 ± 37.5 | 103.8 ± 36 | 97.6 ± 36.8 | 0.3 |
BR, Black race; W, White race; CsA-SM, cyclosporine A and steroid minimization; H, Hispanic; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, North African; PRA, panel-reactive antibodies; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
P < 0.05 CsA-SM versus TAC-SW.
Exposure to Immunosuppressants
Supplementary Table S1 shows the tacrolimus and CsA trough levels in each arm throughout the study, which remained slightly above the planned range in the first 3 months. There were no significant differences in tacrolimus levels between Tac-SW and Tac-SM arms. At study end, 85.4% of Tac-SW, 49% of Tac-SM, and 34.2% of CsA-SM patients were free of corticosteroids. Mean cumulative prednisolone equivalent doses were as follows: Tac-SW 1163 ± 1064, Tac-SM 2210 ± 1000, and CsA-SM 2620 ± 1084 mg (Tac-SW vs. Tac-SM or CsA-SM, P < 0.001).
MMF doses were significantly higher in the CsA arm throughout the study (Tac-SW 0.95 ± 0.3, Tac-SM 0.97 ± 0.3, and CsA-SM 1.4 ± 0.4 g/d at study end; P < 0.001).
Primary Efficacy Variable
Early Alterations in Glucose Metabolism
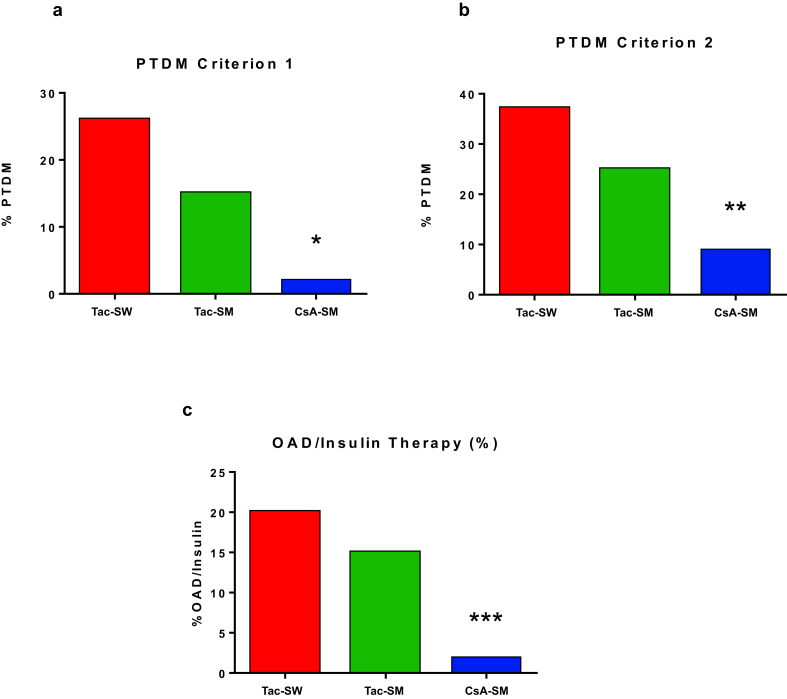
The proportion of patients requiring insulin therapy during their index hospitalization was 31.0%, 35.7%, and 26.2% for Tac-SW, Tac-SM, and CsA-SM arms, respectively (P = 0.5). From the first days after transplantation to the third month, fasting glucose levels were significantly lower in the CsA-SM arm versus Tac-SW or Tac-SM arms (Figure 2a). At 3 months, the proportion of patients with PTDM according to criterion 1 or 2 was lower in CsA-SM arm versus Tac-SW and Tac-SM arms, although of borderline significance (Figure 2b and c).
Figure 2.
Glucose homeostasis alterations 3 months after transplantation in each study arm. (a) Fasting plasma glucose. (b) Proportion of patients with posttransplant diabetes (PTDM) according to criterion 1. (c) Proportion of patients with PTDM according to criterion 2. CsA-SM, cyclosporine A and steroid minimization; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal. *P < 0.05 CsA-SM versus Tac-SW or Tac-SM; **P < 0.05 CsA-SM versus Tac-SW.
Alterations in Glucose Metabolism at Study End
The proportion of patients with PTDM according to criterion 1 or 2 was lower in the CsA-SM arm (Table 2 and Figure 3a and b). Four patients in the Tac-SW, 4 in the Tac-SM, and 7 in the CsA-SM arm, were excluded from criterion 2 because an OGTT could not be performed. The major differences were observed between Tac-SW versus CsA-SM arms (RR 10.2 [1.4–75.3]; P = 0.004 for criterion 1; and RR 3.9 [1.2–12.4]; P = 0.01 for criterion 2). Differences between Tac-SM and CsA-SM did not reach statistical significance (RR 5.8 [0.7–46.3]; P = 0.1 for criterion 1; and RR 2.7 [0.8–8.9]; P = 0.1 for criterion 2). Fewer patients required treatment with hypoglycemic drugs in the CsA-SM arm, although of borderline significance (Table 2 and Figure 3c).
Table 2.
Metabolic, cardiovascular, and renal function data in each arm at study end (1 year)
| Variable | Tac-SW (n = 41) | Tac-SM (n = 39) | CsA-SM (n = 38) | P |
|---|---|---|---|---|
| BMI (kg/m2) | 27.3 ± 4 | 27.9 ± 4 | 28.1 ± 4.6 | 0.6 |
| Weight increase from baseline (kg) | 0.7 ± 7 | -0.2 ± 6 | 1.5 ± 6.7 | 0.5 |
| Fasting glucose (mg/dl) | 104.2 ± 17.5 | 100.6 ± 14.7 | 96.8 ± 16.1 | 0.1 |
| HbA1c (%) | 5.7 ± 0.5 | 5.8 ± 0.5 | 5.5 ± 0.4 | 0.1 |
| Insulin sensitivity (McAuley Index) | 4.7 ± 1.4 | 4.5 ± 1.1 | 4.7 ± 1.2 | 0.7 |
| PTDM (%): | ||||
| -Criterion 1 (fasting glucose, HbA1c; medication use) | 11/41 (26.8) | 6/39 (15.4) | 1/38 (2.6) | 0.01 |
| -Criterion 2: criterion 1 + unmasked PTDM | 14/37 (37.8) | 9/35 (25.7) | 3/31 (9.7) | 0.03 |
| Oral antidiabetics and/or insulin therapy (%) | 8 (20) | 6 (15.4) | 1 (2.6) | 0.06 |
| Insulin therapy | 2 (5) | 1 (2.6) | 0 | 0.4 |
| Acute rejection (%) | 5/44 (11.4) | 2/42 (4.8) | 9/42 (21.4) | 0.07 |
| Corticoresistant acute rejection (%) | 1/44 (2.3) | 1/42 (2.4) | 4/42 (9.5) | 0.2 |
| Serum creatinine (mg/dl) | 1.6 ± 0.7 | 1.5 ± 0.5 | 1.7 ± 0.6 | 0.3 |
| eGFR (ml/min per 1.73 m2) | 51.9 ± 21 | 47.4 ± 14 | 44.6 ± 21 | 0.2 |
| Proteinuria (mg/d) | 208 ± 269 | 241 ± 387 | 343.2 ± 558 | 0.4 |
| Triglycerides (mg/dl) | 159.4 ± 94 | 145.6 ± 53 | 160.8 ± 84 | 0.7 |
| Total cholesterol (mg/dl) | 169.1 ± 31 | 178.2 ± 33.6 | 169 ± 33.4 | 0.4 |
| HDL cholesterol (mg/dl) | 44.8 ± 14 | 49.3 ± 16.9 | 48.4 ± 16.6 | 0.5 |
| LDL cholesterol (mg/dl) | 94 ± 27 | 95.4 ± 26.5 | 88.7 ± 25.7 | 0.5 |
| Patients on ACEI/ARA (%) | 19 (46) | 17 (43.5) | 15 (39.5) | 0.8 |
| Patients on statins (%) | 23 (56) | 24 (61.5) | 28 (78) | 0.2 |
| Patients on ASA (%) | 22 (54) | 19 (49) | 20 (53) | 0.9 |
| Patients on beta-blockers (%) | 16 (39) | 18 (46) | 21 (55) | 0.4 |
| Systolic BP (mm Hg) | 135.4 ± 16 | 134 ± 14 | 136.3 ± 17 | 0.8 |
| Diastolic BP (mm Hg) | 76.7 ± 8.9 | 74.6 ± 9.8 | 76.6 ± 10.3 | 0.6 |
ACEI, angiotensin-converting enzyme inhibitor; ARA, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; CsA-SM, cyclosporine A and steroid minimization; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTDM posttransplant diabetes mellitus; Tac-SM, Tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
Figure 3.
Glucose homeostasis alterations 12 months after transplantation (end of study) in each study arm. (a) Proportion of patients with posttransplant diabetes (PTDM) according to criterion 1. (b) Proportion of patients with PTDM according to criterion 2. (c) Proportion of patients requiring treatment with hypoglycemic drugs. CsA-SM, cyclosporine A and steroid minimization; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal. *P < 0.01; **P < 0.05; ***P = 0.06.
There were no significant differences between arms in weight gain, fasting blood glucose, HbA1c levels, or insulin sensitivity index (Table 2). Blood pressure, lipid levels, and the proportion of patients being treated with statins, renin-angiotensin system inhibitors, acetylsalicylic acid, or beta-blockers were comparable in all 3 arms (Table 2).
The per-protocol analysis showed similar results. The incidence of PTDM at study end was 35% (14/40), 25% (9/36), and 7.7% (2/26), in Tac-SW, Tac-SM, and CsA-SM arms, respectively (P = 0.04). Treatment with hypoglycemic drugs was required in 20.0%, 16.7%, and 3.8% of patients in Tac-SW, Tac-SM, and CsA-SM arms, respectively (P = 0.1).
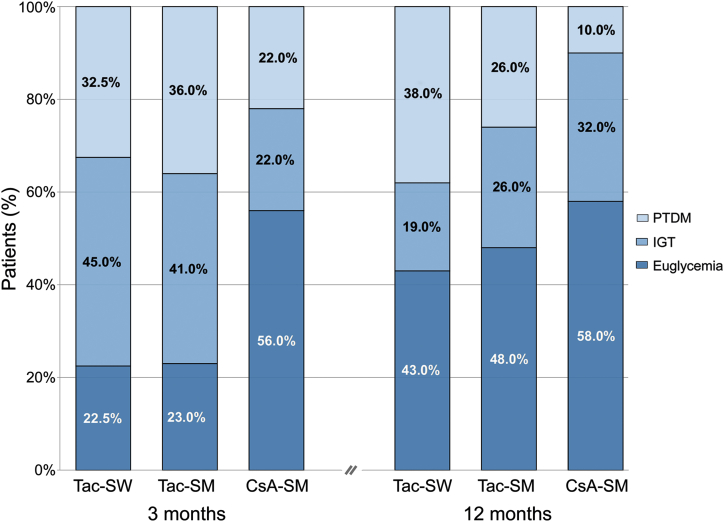
Changes in Glucose Metabolism Between 3 and 12 Months
Compared with 3 months, the proportion of patients with euglycemia increased at 12 months in all arms (Figure 4). In the Tac-SW arm, the proportion of patients with glucose intolerance decreased but that of PTDM did not change. In the Tac-SM arm, both glucose intolerance and PTDM diminished. In the CsA-SM arm, PTDM diminished by improving to glucose intolerance at study end.
Figure 4.
Evolution of glucose homeostasis alterations in each study arm from 3 to 12 months after transplantation. CsA-SM, cyclosporine A and steroid minimization; IGT, impaired glucose tolerance; PTDM, posttransplant diabetes; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
Glucose Metabolism After Conversion From CsA to Tacrolimus
A conversion was performed in 9 patients after biopsy-proven acute rejection, 5 in the first 3 months (Supplementary Table S2). None had PTDM at the time of conversion by criterion 1 and only 1 case showed PTDM at study end (Supplementary Table S2).
Glucose Metabolism After Conversion From Calcineurin Inhibitors to Mammalian Target of Rapamycin Inhibitors
A conversion was performed in 1 patient from the Tac-SW arm, 3 from Tac-SM, and 3 from CsA-SM, all to everolimus (Supplementary Table S3). In 5 cases, conversion was due to impaired renal function and interstitial fibrosis and tubular atrophy lesions in the graft biopsy, with no evidence of acute rejection; in 2 cases, conversion was performed after a malignancy was diagnosed (squamous cell carcinoma and non-Hodgkin lymphoma, respectively). After conversion, no patient developed PTDM (Supplementary Table S3).
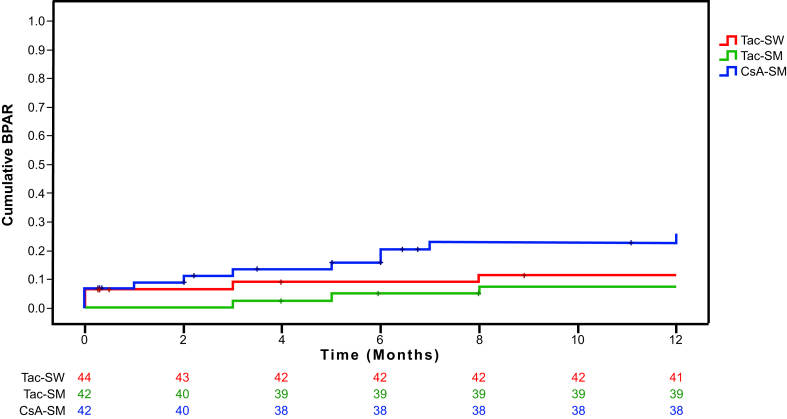
Acute Rejection
The proportion of patients in the CsA-SM arm with acute rejection was higher than in the Tac-SW and Tac-SM arms (Table 2). A total of 3 patients had 2 episodes of biopsy-proven acute rejection during the study, 1 in the Tac-SM arm and 2 in the CsA-SM arm. Thus, the cumulative incidence of acute rejection was significantly higher in the CsA-SM arm (P = 0.04; Figure 5). Steroid-resistant acute rejections requiring treatment with rabbit thymoglobulin were more frequent in the CsA-SM arm (Table 2). The 2 cases of grade II acute rejection and the 2 cases of biopsy-proven acute humoral rejection were in the CsA-SM arm. Only 1 graft was lost due to acute rejection, in the CsA-SM arm.
Figure 5.
Cumulative incidence of biopsy-proven acute rejection (BPAR) in each study arm. Log-rank test P = 0.04. CsA-SM, cyclosporine A and steroid minimization; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
Graft and Patient Survival
Uncensored-death graft survival was similar between study arms (Supplementary Figure S1A). During the study, a total of 10 grafts were lost: 3 in the Tac-SW arm (hemolytic uremic syndrome n = 1, death n = 2); 3 in the Tac-SM arm (graft thrombosis n = 1, pyelonephritis of the graft n = 1, death n = 1); and 4 in the CsA-SM arm (graft thrombosis n = 1, acute rejection n = 1, death n = 2).
Patient survival was similar in all 3 arms (Supplementary Figure S1B). During the study, 5 patients died: 2 in the Tac-SW arm (sudden death 1, cytomegalovirus pneumonia 1), 1 in the Tac-SM arm (shock of uncertain origin), and 2 in the CsA-SM arm (sudden death 1, Pseudomonas aeruginosa pneumonia plus sepsis 1).
Renal Function
Estimated glomerular filtration rate and proteinuria at the end of the study were not different between arms (Table 2).
Adverse Events
Table 3 summarizes the adverse effects. A trend to a higher incidence of acute graft pyelonephritis in the CsA-SM arm was observed (P = 0.07). However, the overall incidence of infectious events and proportion of affected patients were not different among the 3 arms. The proportion of patients with severe adverse events (those shown in Table 3 plus death, graft loss, and biopsy-proven acute rejection) was comparable in the Tac-SW and Tac-SM arms, but significantly higher in the CsA-SM arm (38.6%, 42.9%, and 71.4%, respectively; P = 0.005).
Table 3.
Number of adverse events and proportion of affected/at-risk patients at study end
| Tac-SW | Tac-SM | CsA-SM | Total | P | |
|---|---|---|---|---|---|
| Cardiovascular | |||||
| Sudden death | 1 | 0 | 1 | 2 | 0.6 |
| IHD | 0 | 1 | 2 | 3 | 0.3 |
| Stroke | 0 | 1 | 0 | 1 | 0.3 |
| Total no. cardiovascular events | 1 | 2 | 3 | 6 | 0.6 |
| Patients affected/at risk (%) | 1/44 (2.3) | 2/42 (4.8) | 3/42 (7.1) | 6/128 (4.7) | 0.6 |
| Infections | |||||
| Pneumonia | 1 | 1 | 2 | 4 | 0.8 |
| CMV | 7 | 6 | 12 | 25 | 0.2 |
| BKV | 4 | 2 | 3 | 9 | 0.7 |
| Acute pyelonephritis | 2 | 6 | 1 | 9 | 0.07 |
| Bacteriemia | 1 | 5 | 2 | 8 | 0.2 |
| Total no. infectious events | 15 | 20 | 20 | 55 | 0.3 |
| Patients affected/at risk (%) | 10/44 (22.7) | 11/42 (26.2) | 14/42 (33.3) | 35/128 (27.3) | 0.5 |
| Neoplasia | |||||
| Native kidney | 0 | 1 | 0 | 1 | 0.4 |
| PTLD | 0 | 0 | 1 | 1 | 0.4 |
| Squamous carcinoma | 0 | 0 | 1 | 1 | 0.4 |
| Total no. neoplasia | 0 | 1 | 2 | 3 | 0.3 |
| Patients affected/at risk (%) | 0/44 (0) | 1/42 (2.4) | 2/42 (4.8) | 3/128 (7.1) | 0.3 |
BKV, BK virus infection; CMV, cytomegalovirus infection; CsA-SM, cyclosporine A and steroid minimization; IHD, ischemic heart disease; PTLD, posttransplant lymphoproliferative disease; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
Discussion
This study shows that in patients with a high risk for PTDM, tacrolimus-based immunosuppression, administered with basiliximab and MMF plus SM, provides the best balance between PTDM and acute rejection incidence. Under a similar regimen, rapid SW did not further reduce the incidence of PTDM and was associated with a slight increase in biopsy-proven acute rejection (BPAR). CsA-based immunosuppression with SM was associated with a larger decrease in PTDM but at the expense of increasing BPAR that led to the interruption of the trial.
This randomized controlled trial was designed to balance the risk-benefit associated with the use of steroids, tacrolimus, or cyclosporin A in high-risk patients for PTDM. Based on previous reports showing that SW reduced the risk for PTDM,20, 21, 22 we aimed to test whether a rapid SW at day 8 was superior to SM in terms of PTDM incidence without affecting efficacy defined by BPAR rate. The inclusion of a CsA group was based on the established lower beta-cell toxicity of CsA in a situation of insulin resistance.16, 26, 27, 28 We chose a CsA dose between the standard and low-dose groups of the Symphony trial,38 plus basiliximab. Thus, we could test whether the use of CsA in a situation of older recipients and immunosenescense39 decreases the incidence of PTDM without affecting efficacy.
Mean tacrolimus and CsA levels were in the upper limit of the planned range, especially during the first 3 months, which might reflect the conservative approach of investigators in this type of study (Supplementary Table S1).
A relevant finding of this study is that in patients at high risk for developing PTDM, tacrolimus-based immunosuppression with SM results in a lower than anticipated 1-year incidence of PTDM with a low rate of BPAR. The observed 26% incidence compares favorably with the reported 44% to 56% in high-risk patients,15, 16 and falls within the 17% to 39% observed in the transplant population at large.16, 22, 40 Additionally, PTDM was manageable because only 1 patient required insulin therapy at study end and the mean HbA1c was <7% (6.4 ± 0.6; 95% confidence interval 5.7%–7.0%). Finally, the consistent improvement in glucose metabolism from 3 to 12 months due to decreasing immunosuppression (Figure 4) underscores the partial reversibility of tacrolimus-induced dysglycemia.4, 29, 30, 31
Compared with SM, rapid SW in tacrolimus-treated patients did not provide a further reduction of PTDM. The associated slight increase in BPAR (11.4% vs. 4.8%), a specific risk factor of PTDM due to the concomitant use of high corticosteroid doses,1, 2, 3, 40 may at least partly explain this finding. In fact, 3 of the 5 patients with BPAR developed PTDM. Furthermore, withdrawal of 5 mg/d of prednisone did not improve the insulin sensitivity index in RT recipients.41 Indeed, insulin sensitivity index values at study end were comparable between arms (Table 2).
Although in the population at large, CsA is associated with a lower risk of PTDM than tacrolimus,25, 38 this is the first study showing the superiority of CsA in high-risk recipients (Table 2). This was observed from the first weeks of the study (Figure 2), therefore minimizing the exposure to early glucotoxicity, a risk factor for PTDM at 1 year.42 At study end, the 21.4% rate of BPAR was slightly lower than the 24% to 26% observed in the CsA arms of the Symphony trial.38 However, it was at least twice that of the tacrolimus arms (Table 2, Figure 5), leading to the interruption of the trial. CsA exposure in the present study, especially in the early posttransplant period, was lower than in controlled trials, showing a similar efficacy between tacrolimus and CsA.25, 43 Furthermore, SM in this context may have added a further risk of underimmunosuppression. A contributing factor to the suboptimal efficacy of CsA in our aging population may have been the higher immunogenicity of a consistent proportion of grafts belonging to expanded criteria donors with delayed graft function39 (Table 1).
Although conversion from CsA to tacrolimus in prevalent RT recipients does not affect glucose metabolism,44, 45 the effect of early conversion is not well established. In this study, a conversion was performed in 9 patients, 5 during the first 3 months (Supplementary Table S2). Interestingly, only 1 patient developed PTDM 10 months later. This finding is in agreement with the results of a prospective randomized trial comparing CsA and tacrolimus46 showing a 48% reduction of insulin secretion at week 3 in patients receiving tacrolimus, and this divergence disappeared from 3 weeks onward. Therefore, protecting the beta-cell of high-risk patients during the first month by the temporal substitution of tacrolimus by CsA, under rabbit thymoglobulin or at a higher exposure than the present study, or the use of new regimens facilitating early lower exposure to tacrolimus, may further decrease the incidence of PTDM without decreasing efficacy. Alternatively, and based on the results of a recent randomized trial,31 a tacrolimus-based regimen with SM may be used to prevent acute rejection during the early posttransplant period and to replace tacrolimus with CsA in patients with inadequately controlled PTDM in the maintenance phase. However, this should be demonstrated in future studies with an ad hoc design.
Adverse events were not significantly different between the study arms (Table 3). A nonsignificant trend to increased acute graft pyelonephritis and secondary urosepsis was observed in the tacrolimus with SM arm. Unfortunately, differences in other confounding factors, like days of indwelling bladder catheterization or double-J stents, could not be determined; however, the total number of infections was comparable among arms. The recruited cohort showed a relatively high incidence of graft loss and patient death by current standards but this has to be interpreted in the context of a mean recipient age of 60 years and a high proportion of expanded criteria donor and delayed graft function (Table 1).
This study has limitations. First, the recruitment was prematurely stopped for safety reasons. Therefore, the study was not powered to demonstrate the observed noninferiority of Tac-SM arm versus Tac-SW in terms of PTDM. Thus, the comparison between these arms should be taken with caution. Second, because an OGTT at the time of transplantation could not be performed, we may have missed 3% to 8% of unrecognized diabetes that could be higher in our at-risk population.47, 48 A pretransplant OGTT to rule out occult diabetes could have been of relevance, but it is impractical in a deceased-donor setting. However, we adopted the term PTDM avoiding new-onset diabetes, to simply describe persistent posttransplant hyperglycemia not present at transplantation.49 In fact, differences between arms in posttransplant hyperglycemia (criterion 1) were similar to that observed after including the OGTT (criterion 2) (Table 2). Our population was mainly white and of low immunological risk, making the results not representative of other transplant populations. Finally, donor-specific antibody data were not planned when the study was designed and thus were not widely collected for patients with BPAR.
In conclusion, tacrolimus-based immunosuppression, administered with basiliximab and MMF plus SM, provides the best balance between PTDM and acute rejection incidence in high-risk patients. Rapid SW or CsA-based immunosuppression showed no advantages or increased the risk of acute rejection.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was entirely funded by a grant from the Instituto de Salud Carlos III, Madrid, Spain (FIS EC 08/00055; RedInRen Network RD16/0009/0031, RD16/0009/0006 and RD16/0009/0030) and FONDOS FEDER. We are grateful to the Research Unit-UCICEC of the Hospital Universitario de Canarias for the support developing the trial. We are also extremely grateful to the kidney transplant teams of the hospitals involved and thank Ian Johnstone for help with English language editing. This trial is registered with ClinicalTrials.gov, number NCT 01002339.
Footnotes
AT and DH contributed equally to the study.
Table S1. Trough blood levels of tacrolimus and cyclosporine A (CsA) at each study point.
Table S2. Glucose metabolism before and after conversion from cyclosporine A (CsA) or tacrolimus to everolimus.
Table S3. Glucose metabolism before and after conversion from cyclosporine A (CsA) or tacrolimus to everolimus.
Figure S1. Noncensored graft (A) and patient survival (B) in each study arm. Log-rank test P = 0.9 and P = 0.8, respectively. CsA-SM, cyclosporine A and steroid minimization; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Trough blood levels of tacrolimus and cyclosporine A (CsA) at each study point.
Glucose metabolism before and after conversion from cyclosporine A (CsA) or tacrolimus to everolimus.
Glucose metabolism before and after conversion from cyclosporine A (CsA) or tacrolimus to everolimus.
Figure S1.
Noncensored graft (A) and patient survival (B) in each study arm. Log-rank test P = 0.9 and P = 0.8, respectively. CsA-SM, cyclosporine A and steroid minimization; Tac-SM, tacrolimus and steroid minimization; Tac-SW, tacrolimus and rapid steroid withdrawal.
References
- 1.Sharif A., Cohney S. Post-transplantation diabetes-state of the art. Lancet Diabetes Endocrinol. 2016;4:337–349. doi: 10.1016/S2213-8587(15)00387-3. [DOI] [PubMed] [Google Scholar]
- 2.Yates C.J., Fourlanos S., Hjelmesaeth J. New-onset diabetes after kidney transplantation-changes and challenges. Am J Transplant. 2012;12:820–828. doi: 10.1111/j.1600-6143.2011.03855.x. [DOI] [PubMed] [Google Scholar]
- 3.Hecking M., Werzowa J., Haidinger M. Novel views on new-onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant. 2013;28:550–566. doi: 10.1093/ndt/gfs583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porrini E.L., Díaz J.M., Moreso F. Clinical evolution of post-transplant diabetes mellitus. Nephrol Dial Transplant. 2016;31:495–505. doi: 10.1093/ndt/gfv368. [DOI] [PubMed] [Google Scholar]
- 5.Wauters R.P., Cosio F.G., Suarez Fernandez M.L. Cardiovascular consequences of new onset hyperglycemia after kidney transplantation. Transplantation. 2012;94:377–382. doi: 10.1097/TP.0b013e3182584831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valderhaug T.G., Hjelmesæth J., Hartmann A. The association of early post-transplant glucose levels with long-term mortality. Diabetologia. 2011;54:1341–1349. doi: 10.1007/s00125-011-2105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dienemann T., Fujii N., Li Y. Long-term patient survival and kidney allograft survival in post-transplant diabetes mellitus: a single-center retrospective study. Transpl Int. 2016;29:1017–1028. doi: 10.1111/tri.12807. [DOI] [PubMed] [Google Scholar]
- 8.Woodward R.S., Schnitzler M.A., Baty J. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 9.OʼConnell P.J., Kuypers D.R., Mannon R.B. Clinical trials for immunosuppression in transplantation: the case for reform and change in direction. Transplantation. 2017;101:1527–1534. doi: 10.1097/TP.0000000000001648. [DOI] [PubMed] [Google Scholar]
- 10.Shivaswamy V., Boerner B., Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37:37–61. doi: 10.1210/er.2015-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer N.D., Cochetti P.T., Anil Kumar M.S. Association of metabolic syndrome with development of new-onset diabetes after transplantation. Transplantation. 2010;90:861–866. doi: 10.1097/TP.0b013e3181f1543c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasiske B.L., Snyder J.J., Gilbertson D., Matas A.J. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 13.Shah T., Kasravi A., Huang E. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82:1673–1676. doi: 10.1097/01.tp.0000250756.66348.9a. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin T., Reaven G., Abbasi F. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 15.Chakkera H.A., Chang Y.H., Ayub A. Validation of a pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care. 2013;36:2881–2886. doi: 10.2337/dc13-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porrini E., Delgado P., Alvarez A. The combined effect of pre-transplant triglyceride levels and the type of calcineurin inhibitor in predicting the risk of new onset diabetes after renal transplantation. Nephrol Dial Transplant. 2008;23:1436–1441. doi: 10.1093/ndt/gfm762. [DOI] [PubMed] [Google Scholar]
- 17.Cosio F.G., Pesavento T.E., Kim S. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62:1440–1446. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghisdal L., Van Laecke S., Abramowicz M.J. New-onset diabetes after renal transplantation: risk assessment and management. Diabetes Care. 2012;35:181–188. doi: 10.2337/dc11-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham P.T., Pham P.M., Pham S.V. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175–186. doi: 10.2147/DMSO.S19027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boots J.M., Christiaans M.H., Van Duijnhoven E.M. Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: a pilot study. Transplantation. 2002;74:1703–1709. doi: 10.1097/00007890-200212270-00011. [DOI] [PubMed] [Google Scholar]
- 21.Knight S.R., Morris P.J. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1–14. doi: 10.1097/TP.0b013e3181c518cc. [DOI] [PubMed] [Google Scholar]
- 22.Thomusch O., Wiesener M., Opgenoorth M. Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open-label, multicentre, randomised controlled trial. Lancet. 2016;388:3006–3016. doi: 10.1016/S0140-6736(16)32187-0. [DOI] [PubMed] [Google Scholar]
- 23.Pirsch J.D., Henning A.K., First M.R. New-onset diabetes after transplantation: results from a double-blind early corticosteroid withdrawal trial. Am J Transplant. 2015;15:1982–1990. doi: 10.1111/ajt.13247. [DOI] [PubMed] [Google Scholar]
- 24.Øzbay L.A., Smidt K., Mortensen D.M. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br J Pharmacol. 2011;162:136–146. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincenti F., Friman S., Scheuermann E. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 26.Neylan J. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation. 1998;65:515–523. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 27.Bloom R.D., Rao V., Weng F. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13:1374–1380. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Rodriguez A.E., Triñanes J., Velazquez-Garcia S. The higher diabetogenic risk of tacrolimus depends on pre-existing insulin resistance. A study in obese and lean Zucker rats. Am J Transplant. 2013;13:1665–1675. doi: 10.1111/ajt.12236. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Rodríguez A.E., Triñanes J., Porrini E. Glucose homeostasis changes and pancreatic β-cell proliferation after cyclosporin in tacrolimus-induced diabetes mellitus. Nefrologia. 2015;35:264–272. doi: 10.1016/j.nefro.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Ghisdal L., Bouchta N.B., Broeders N. Conversion from tacrolimus to cyclosporine A for new-onset diabetes after transplantation: a single-centre experience in renal transplanted patients and review of the literature. Transpl Int. 2008;21:146–151. doi: 10.1111/j.1432-2277.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- 31.Wissing K.M., Abramowicz D., Weekers L. Prospective randomized study of conversion from tacrolimus to cyclosporine A to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. Am J Transplant. 2018;18:1726–1734. doi: 10.1111/ajt.14665. [DOI] [PubMed] [Google Scholar]
- 32.Solez K., Colvin R.B., Racusen L.C. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 33.Davidson J., Wilkinson A., Dantal J. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(Suppl 10):3–24. doi: 10.1097/01.TP.0000069952.49242.3E. [DOI] [PubMed] [Google Scholar]
- 34.Porrini E., Bayes B., Diaz J.M. Hyperinsulinemia and hyperfiltration in renal transplantation. Transplantation. 2009;87:274–279. doi: 10.1097/TP.0b013e318191a7d5. [DOI] [PubMed] [Google Scholar]
- 35.Oterdoom L.H., de Vries A.P., van Son W.J. Validation of insulin resistance indexes in a stable renal transplant population. Diabetes Care. 2005;28:2424–2429. doi: 10.2337/diacare.28.10.2424. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association Standards of medical care in diabetes 2010. Diabetes Care. 2010;33(Suppl 1):11–61. [Google Scholar]
- 37.Hernández D., Miquel R., Porrini E. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine-based immunosuppression. Transplantation. 2007;84:706–714. doi: 10.1097/01.tp.0000282872.17024.b7. [DOI] [PubMed] [Google Scholar]
- 38.Ekberg H., Tedesco-Silva H., Demirbas A. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 39.Krenzien F., ElKhal A., Quante M. A rationale for age-adapted immunosuppression in organ transplantation. Transplantation. 2015;99:2258–2268. doi: 10.1097/TP.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mourad G., Glyda M., Albano L. Incidence of posttransplantation diabetes mellitus in de novo kidney transplant recipients receiving prolonged-release tacrolimus-based immunosuppression with 2 different corticosteroid minimization strategies: ADVANCE, a randomized controlled trial. Transplantation. 2017;101:1924–1934. doi: 10.1097/TP.0000000000001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Midtvedt K., Hjelmesaeth J., Hartmann A. Insulin resistance after renal transplantation: the effect of steroid dose reduction and withdrawal. J Am Soc Nephrol. 2004;15:3233–3239. doi: 10.1097/01.ASN.0000145435.80005.1E. [DOI] [PubMed] [Google Scholar]
- 42.Hecking M., Haidinger M., Döller D. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23:739–749. doi: 10.1681/ASN.2011080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva H.T., Jr., Yang H.C., Abouljoud M. One-year results with extended-release tacrolimus/MMF, tacrolimus/MMF and cyclosporine/MMF in de novo kidney transplant recipients. Am J Transplant. 2007;7:595–608. doi: 10.1111/j.1600-6143.2007.01661.x. [DOI] [PubMed] [Google Scholar]
- 44.Gelens M.A., Christiaans M.H., van Hooff J.P. Glucose metabolism before and after conversion from cyclosporine microemulsion to tacrolimus in stable renal recipients. Nephrol Dial Transplant. 2008;23:701–706. doi: 10.1093/ndt/gfm544. [DOI] [PubMed] [Google Scholar]
- 45.Luan F.L., Zhang H., Schaubel D.E. Comparative risk of impaired glucose metabolism associated with cyclosporine versus tacrolimus in the late posttransplant period. Am J Transplant. 2008;8:1871–1877. doi: 10.1111/j.1600-6143.2008.02328.x. [DOI] [PubMed] [Google Scholar]
- 46.van Duijnhoven E.M., Christiaans M.H., Boots J.M. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol. 2002;13:213–220. doi: 10.1681/ASN.V131213. [DOI] [PubMed] [Google Scholar]
- 47.Bergrem H.A., Valderhaug T.G., Hartmann A. Undiagnosed diabetes in kidney transplant candidates: a case-finding strategy. Clin J Am Soc Nephrol. 2010;5:616–622. doi: 10.2215/CJN.07501009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caillard S., Eprinchard L., Perrin P. Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: role of systematic screening by oral glucose tolerance test. Transplantation. 2011;91:757–764. doi: 10.1097/TP.0b013e31820f0877. [DOI] [PubMed] [Google Scholar]
- 49.Sharif A., Hecking M., de Vries A.P. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trough blood levels of tacrolimus and cyclosporine A (CsA) at each study point.
Glucose metabolism before and after conversion from cyclosporine A (CsA) or tacrolimus to everolimus.
Glucose metabolism before and after conversion from cyclosporine A (CsA) or tacrolimus to everolimus.