Abstract
Abrupt disease onset and severe metabolic disorders are main characteristics of fulminant type 1 diabetes. Diffusion-weighted magnetic resonance imaging (DWI) is an imaging technique that reflects restricted diffusion in organs and can detect mononuclear cell infiltration into the pancreas at the onset of the disease. Fourteen patients with fulminant type 1 diabetes who underwent abdominal magnetic resonance imaging were recruited for the measurement of apparent diffusion coefficient (ADC) values of the pancreas that were compared with those of 21 non-diabetic controls. The ADC values of all parts of the pancreas were significantly lower in fulminant type 1 diabetes than in controls (head, 1.424 ± 0.382 × 10−3 vs. 1.675 ± 0.227 × 10−3 mm2/s; body, 1.399 ± 0.317 × 10−3 vs. 1.667 ± 0.170 × 10−3 mm2/s; tail, 1.336 ± 0.247 × 10−3 vs. 1.561 ± 0.191 × 10−3 mm2/s; mean, 1.386 ± 0.309 × 10−3 vs. 1.634 ± 0.175 × 10−3 mm2/s) (p < 0.01). The best cut-off value indicated that the sensitivity was 86% and the specificity was 71% when using DWI, which was also efficient in two atypical patients with fulminant type 1 diabetes without elevated levels of exocrine pancreatic enzymes or with high HbA1c levels due to the preexistence of type 2 diabetes. The ADC values were significantly correlated to plasma glucose levels and arterial pH, and tended to increase with the lapse of time. DWI may be an additional tool for making an efficient diagnosis of fulminant type 1 diabetes.
Keywords: Fulminant, Type 1 diabetes, Magnetic resonance imaging, Diffusion-weighted imaging, Apparent diffusion coefficient
Introduction
Type 1 diabetes mellitus is characterized by an absolute insulin deficiency derived from beta-cell destruction [1, 2]. Fulminant type 1 diabetes accounts for 20% of acute onset type 1 diabetes in Japan and recent reports have revealed that this subtype is a major type in other East Asian countries such as Korea and China [3, 4]. This disease is life threatening and is characterized by an extremely abrupt onset with ketoacidosis, and, therefore, needs to be diagnosed and treated as soon as possible. Other characteristics of fulminant type 1 diabetes include high plasma glucose levels accompanied with almost normal glycated hemoglobin (HbA1c) levels, absence of islet-related autoantibodies in general, elevated levels of exocrine pancreatic enzymes (up to 98% of patients with fulminant type 1 diabetes), and very low levels of C-peptide [5–8]. However, each of these factors is not specific to fulminant type 1 diabetes. To diagnose fulminant type 1 diabetes more accurately, more specific biochemical, immunological or other markers are recommended.
Diffusion-weighted magnetic resonance imaging (DWI) is an advanced imaging technique that reflects the random movement of water molecules within extracellular spaces and between intracellular and extracellular spaces. Apparent diffusion coefficient (ADC) values, which can be calculated from DWI data, are usually used for the quantitative assessment of water mobility in various organs. DWI was established primarily for the diagnosis of acute stroke and brain tumors, which impair water mobility [9, 10]. Following recent technical advances, DWI has also been used to detect various disorders of abdominal organs, such as liver fibrosis and Crohn’s disease [11, 12], and there are reports that pancreatic inflammatory diseases such as acute pancreatitis (AP), chronic pancreatitis (CP), and autoimmune pancreatitis (AIP) can be detected by DWI as high intensity areas or as decreased ADC values indicating a restricted diffusion in specific organs resulting from fibrosis, and the infiltration of inflammatory cells [13–19]. If mononuclear cell infiltration into the pancreas at the onset of fulminant type 1 diabetes can be detected by DWI, it will be a useful technique for the diagnosis of disease. In the present study, we compared ADC values of the pancreas between fulminant type 1 diabetes and non-diabetic control subjects, and then examined its potential association to several clinical parameters related to diabetes.
Subjects and methods
Subjects
We retrospectively reviewed the records of patient with fulminant type 1 diabetes registered to the committee of the Japan Diabetes Society from 2001 to 2013. They were diagnosed in accordance with the following inclusion criteria proposed by the Committee of the Japan Diabetes Society: (1) presence of ketosis or ketoacidosis within a week after the onset of hyperglycemic symptoms; (2) plasma glucose level ≥ 16.0 mmol/l (288 mg/dl) and HbA1c level < 8.7%* at first visit (*this value is not applicable for patients with previously diagnosed glucose intolerance); (3) urinary C-peptide excretion < 10 μg/day or fasting serum C-peptide level < 0.3 ng/ml (0.10 nmol/l) and peak serum C-peptide level < 0.5 ng/ml (0.17 nmol/l) after intravenous glucagon (1 mg) injection or a meal load soon after disease onset [20]. Fifty patients underwent abdominal MRI within about 1 month after disease onset (4–40 days) for the purpose of examining the causes of elevated serum levels of exocrine pancreatic enzymes or abdominal symptoms. There were two patients difficult to diagnose with fulminant type 1 diabetes. One patient with type 2 diabetes showed high HbA1c level at the disease onset (8.9%). Another patient lacked definite elevation in exocrine pancreatic enzymes: serum amylase, elastase-1, and lipase levels were 0.25, 0.42, and 0.61 times higher than the upper limit of the normal range, respectively.
Previous studies reported that the ADC values of gray and white matter measured from MRI data are variable among different MRI vendors, although the variability in ADC values from three MRI vendors (Siemens Medical Solutions, Erlangen, Germany; GE Healthcare, Milwaukee, WI, USA; Philips Healthcare, Best, the Netherlands) was relatively small [21]. It was also reported that there was no significant difference among ADC values of the pancreas using 1.5-T MR systems from these three vendors [22]. There were 14 patients enrolled whose MRI data were obtained using these three vendors and that was clear enough to examine (MRI systems are: MAGNETOM avanto and MAGNETOM symphony from Siemens Medical Solutions; SIGNA EXCITE, GENESIS-SIGNA, and SIGNA HDxt from GE Healthcare; ACHIEVA from Philips Healthcare). Especially, MRI data of four patients were from exactly the same MRI systems as non-diabetic control subjects (MAGNETOM Avanto 1.5 T from Siemens Medical Solutions). These 14 patients comprised the study subjects. MRI was performed at 13.7 ± 8.2 days (mean ± SD) after disease onset.
For the non-diabetic control subjects, we enrolled 21 patients from Ikeda Hospital for whom abdominal MRI was performed to examine abdominal diseases except of the pancreas (MRI system is MAGNETOM avanto from Siemens Medical Solutions). The clinical characteristics of the subjects are shown in Table 1.
Table 1.
Clinical characteristics of the study subjects
| Fulminant | Control | p | |
|---|---|---|---|
| n | 14 | 21 | |
| Age (years) | 51 ± 16 | 71 ± 8 | 0.0004 |
| Sex (M/F) | 9/5 | 9/12 | N.S. |
| BMI (kg/m2) | 20.6 ± 3.0 | 21.5 ± 2.5 | N.S. |
| Plasma glucose (mg/dl) | 924 ± 384a | 93 ± 16 | < 0.0001 |
| HbA1c (%) | 6.9 ± 0.9 | 5.0 ± 0.5 | < 0.0001 |
| Arterial pH | 7.14 ± 0.16 | N.D. | – |
| Serum amylaseb | 2.23 ± 2.41 | 0.67 ± 0.25 | 0.0024 |
| GAD Ab-positive (%) | 0 | N.D. | – |
Data are n or mean ± SD
N.D. not determined, M male, F female, BMI body mass index, HbA1c glycated hemoglobin, GAD glutamic acid decarboxylase, Ab antibody
aMeasured at disease onset
bValues are expressed as multiples of the upper limit of the normal range
This study was approved by the ethics committees of the Japan Diabetes Society (Approval No. 22-0015, July 15th, 2010) and Osaka University Hospital (Approval No. 13340, February 2nd, 2014) and carried out in accordance with the Helsinki Declaration of 1964 and later revisions.
MR imaging protocols
For the 21 non-diabetic control subjects, MR imaging examinations were performed using the Magnetom Avanto 1.5 T (Siemens Medical Solutions) with an 18-channel body matrix coil. The DWI sequence was acquired under free breathing with chemical fat saturation [b values, 0 and 800 s/mm2; repetition time (TR), respiratory triggering, data acquired in the end expiration phase; echo time (TE), 70 ms; slice thickness, 7 mm; gap, 25%; number of excitations (NEX), 7].
Examinations of the 14 patients with fulminant type 1 diabetes were performed using commercially available 1.5-T MRI systems from three different vendors: vendor 1 (n = 6) = Siemens Medical Solutions (MAGNETOM avanto and MAGNETOM symphony); vendor 2 (n = 7) = GE Healthcare (SIGNA EXCITE, GENESIS-SIGNA, and SIGNA HDxt); vendor 3 (n = 1) = Philips Healthcare (ACHIEVA). The conditions for DWI were dependent on each institute, except for b values (low b values ranged between 0 and 50 s/mm2, high b values ranged between 600 and 1000 s/mm2). The acquisition parameters of 6 patients with type 1 diabetes using MRI systems from Siemens Medical Solutions are: repetition time (TR), 1700–4099 ms; echo time (TE), 60.0–84.0 ms; slice thickness, 4–8 mm; number of excitations (NEX), 2–8; field of view (FOV), 285–380 mm; matrix, 128 × 72, 128 × 90, 128 × 128, 192 × 115. The acquisition parameters of 7 patients with type 1 diabetes using MRI systems from GE Healthcare are: repetition time (TR), 3000–11250 ms; echo time (TE), 32.4–78.6 ms; slice thickness, 5–8 mm; number of excitations (NEX), 4–10; field of view (FOV), 340–440 mm; matrix, 128 × 128. And the acquisition parameters of 1 patient with type 1 diabetes using MRI systems from Philips Healthcare are: repetition time (TR), 1200 ms; echo time (TE), 49.0 ms; slice thickness, 6 mm; number of excitations (NEX), 4; field of view (FOV), 300 mm; matrix, 112 × 86, 128 × 103. ADC maps were generated on the MR system console and obtained for each slice position.
Assessment
The ADC values were measured by drawing a circular region of interest (ROI) within the pancreas from ADC maps using PACSPLUS Clinic Viewer (PACSPLUS, Tokyo, Japan). Three ROIs were placed in each of the head, body, and tail of the pancreas. The mean of these three ADC values in each part of the pancreas was calculated. Mean ADC values were determined from mean values ADC values in the head, body, and tail of pancreas in each patient.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). The Mann–Whitney U test was used for comparison of ADC values between patients with fulminant type 1 diabetes and non-diabetic control subjects. Receiver operating characteristic (ROC) curves were used to determine the appropriate cut-off values for ADC values to diagnose fulminant type 1 diabetes. The best cut-off values were determined by the point on the curve closest to the (0, 1) point. The correlation between ADC values and clinical data was evaluated by Spearman’s rank correlation coefficient. All statistical analyses were performed with StatView Ver. 5.0 software (SAS Institute Inc., Cary, NC), and statistical significance was defined as p < 0.05.
Results
Comparison of ADC values of pancreas between patients with fulminant type 1 diabetes and non-diabetic control subjects
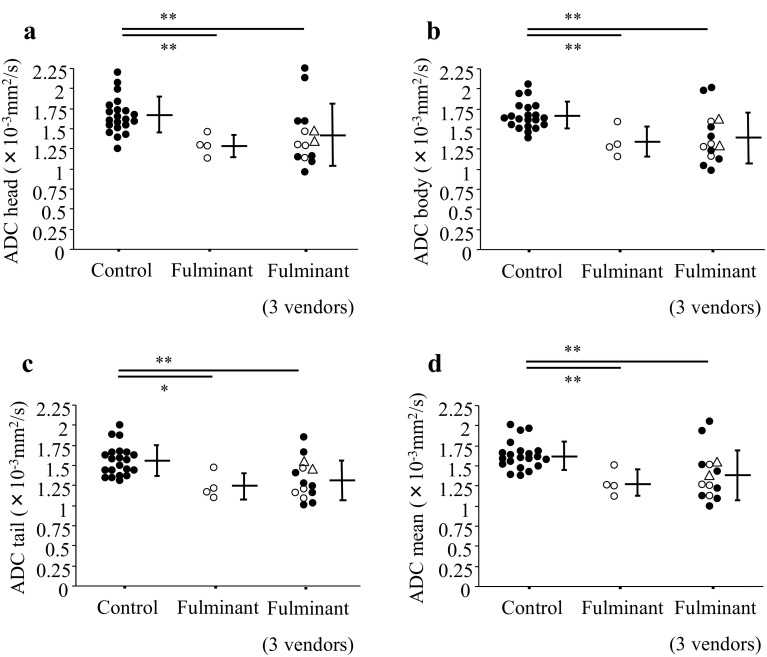
The DWI images and ADC map of a representative case of fulminant type 1 diabetes are shown in Fig. 1. We measured ADC values of the pancreas head, body, and tail in the two groups. Among 14 patients with fulminant type 1 diabetes, we first examined four patients using the same MRI systems and vendors as the control subjects (Magnetom Avanto 1.5 T, Siemens Medical Solutions). The ADC values of patients with fulminant type 1 diabetes were significantly lower than those of non-diabetic control subjects in all parts of the pancreas (head, 1.296 ± 0.137 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.675 ± 0.227 × 10−3 mm2/s for controls, p = 0.0076; body, 1.338 ± 0.185 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.667 ± 0.170 × 10−3 mm2/s for controls, p = 0.0095; tail, 1.243 ± 0.167 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.561 ± 0.191 × 10−3 mm2/s for controls, p = 0.0144; mean, 1.292 ± 0.161 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.634 ± 0.175 × 10−3 mm2/s for controls, p = 0.0061). Then we made a comparison between all 14 patients with fulminant type 1 diabetes using various MRI systems from the three vendors (Siemens Medical Solutions, GE Healthcare, Philips Healthcare) with non-diabetic controls. The ADC values of patients with fulminant type 1 diabetes were significantly lower than those of non-diabetic control subjects in all parts of the pancreas (head, 1.424 ± 0.382 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.675 ± 0.227 × 10−3 mm2/s for controls, p = 0.0064; body, 1.399 ± 0.317 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.667 ± 0.170 × 10−3 mm2/s for controls, p = 0.0034; tail, 1.336 ± 0.247 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.561 ± 0.191 × 10−3 mm2/s for controls, p = 0.0071; mean, 1.386 ± 0.309 × 10−3 mm2/s for fulminant type 1 diabetes vs. 1.634 ± 0.175 × 10−3 mm2/s for controls, p = 0.0024) (Fig. 2).
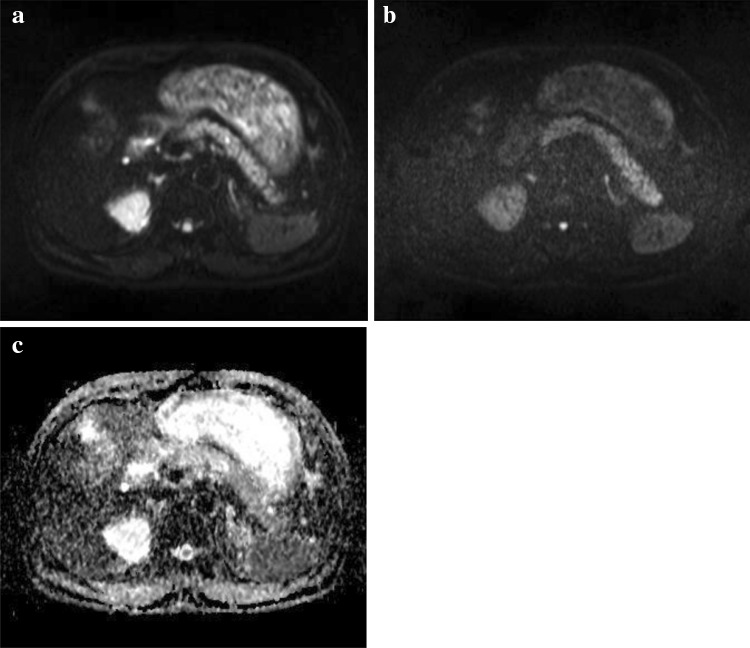
Fig. 1.
DWI images of a representative case of fulminant type 1 diabetes. DWI images of low b value (b = 50 s/mm2) (a), high b value (b = 800 s/mm2) (b), ADC map (c)
Fig. 2.
Comparison of ADC values of pancreas between patients with fulminant type 1 diabetes patients and non-diabetic control subjects. ADC values were measured in each part of the head (a), body (b), and tail (c) of the pancreas. The mean ADC values of three parts were calculated (d). Fulminant type 1 diabetic patients using the same MRI systems as control subjects were indicated by opened circles (Magnetom Avanto 1.5 T from Siemens Medical Solutions). The three vendors include Siemens Medical Solutions, GE Healthcare, and Philips Healthcare. Data are presented as mean ± SD. Two atypical cases of fulminant type 1 diabetes (with type 2 diabetes, without elevation of exocrine pancreatic enzymes, respectively) were indicated by opened triangles. *p < 0.05, **p < 0.01
Optimal cut-off value and areas under the ROC curves of ADC values
In the ROC curves, the best cut-off values for ADC values to diagnose fulminant type 1 diabetes were 1.497 × 10−3 mm2/s for pancreas head (sensitivity 71%, specificity 81%), 1.526 × 10−3 mm2/s for pancreas body (sensitivity 71%, specificity 81%), 1.428 × 10−3 mm2/s for pancreas tail (sensitivity 64%, specificity 76%), and 1.548 × 10−3 mm2/s for the mean of three parts of the pancreas (sensitivity 86%, specificity 71%). Areas under the ROC curves (AUCs) of pancreatic ADC values were 0.776 (95% CI 0.592, 0.959) for pancreas head, 0.796 (95% CI 0.616, 0.976) for pancreas body, 0.772 (95% CI 0.599, 0.945) for pancreas tail, and 0.806 (95% CI 0.631, 0.981) for the mean of the three parts of the pancreas, indicating that they could diagnose fulminant type 1 diabetes with moderate accuracy.
Of note, ADC values of a patient with fulminant type 1 diabetes who was treated for type 2 diabetes were 1.459, 1.615, 1.548 and 1.541 × 10−3 mm2/s for pancreas head, body, tail, and mean, respectively. The head and mean ADC values were slightly lower than the cut-off values. ADC values of a patient without elevation in exocrine pancreatic enzymes were 1.333, 1.293, 1.447, and 1.358 × 10−3 mm2/s for pancreas head, body, tail, and mean, respectively. They are lower than the cut-off values except for pancreatic tail.
Association between ADC values and plasma glucose and arterial pH
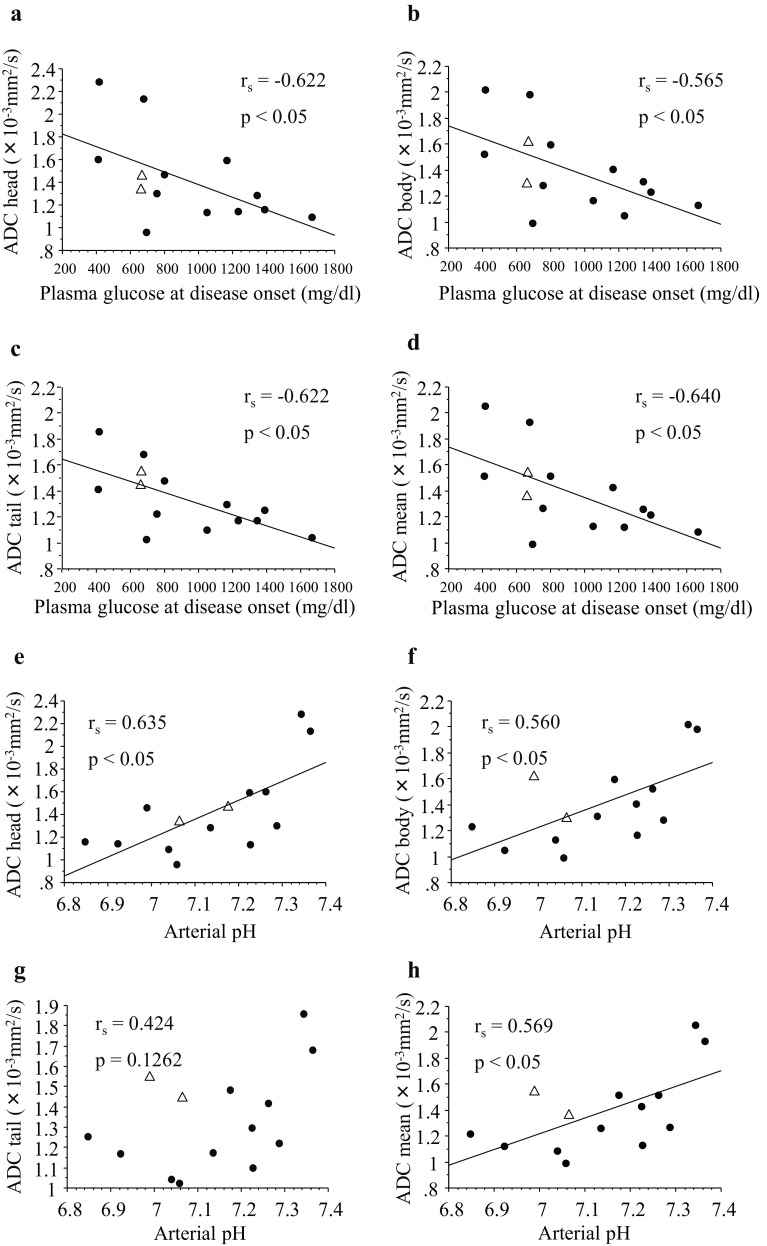
Regarding the fulminant type 1 diabetes group, we examined the association between ADC values and clinical parameters including the number of days taken to perform MRI since the disease onset, age, body mass index, plasma glucose level, HbA1c level, serum amylase, lipase, and elastase-1 levels, as well as arterial pH level at disease onset. The ADC values in all parts of the pancreas and plasma glucose levels at disease onset were significantly correlated (head, rs = − 0.622, p < 0.05; body, rs = − 0.565, p < 0.05; tail, rs = − 0.622, p < 0.05; mean, rs = − 0.640, p < 0.05). The ADC values of pancreas head, body, mean and arterial pH levels were also significantly correlated except for tail (head, rs = 0.635, p < 0.05; body, rs = 0.560, p < 0.05; tail, rs = 0.424, p = 0.126; mean, rs = 0.569, p < 0.05) (Fig. 3). There was no relationship between the ADC values and the other parameters (number of days taken to perform MRI since the disease onset, age, body mass index, HbA1c level, serum amylase, lipase, and elastase-1 levels; data not shown).
Fig. 3.
Correlation between ADC values of patients with fulminant type 1 diabetes and plasma glucose level or arterial pH level at the onset of the disease. They were evaluated in each part of the head (a, e), body (b, f), tail (c, g), and mean (d, h) of the pancreas. Correlation between ADC values and plasma glucose levels (a–d). Correlation between ADC values and arterial pH level (e–h). Two atypical cases of fulminant type 1 diabetes (with type 2 diabetes, without elevation of exocrine pancreatic enzymes, respectively) were indicated by opened triangles. rs = correlation coefficient. A p value < 0.05 indicated a significant difference
Chronological changes in the ADC values
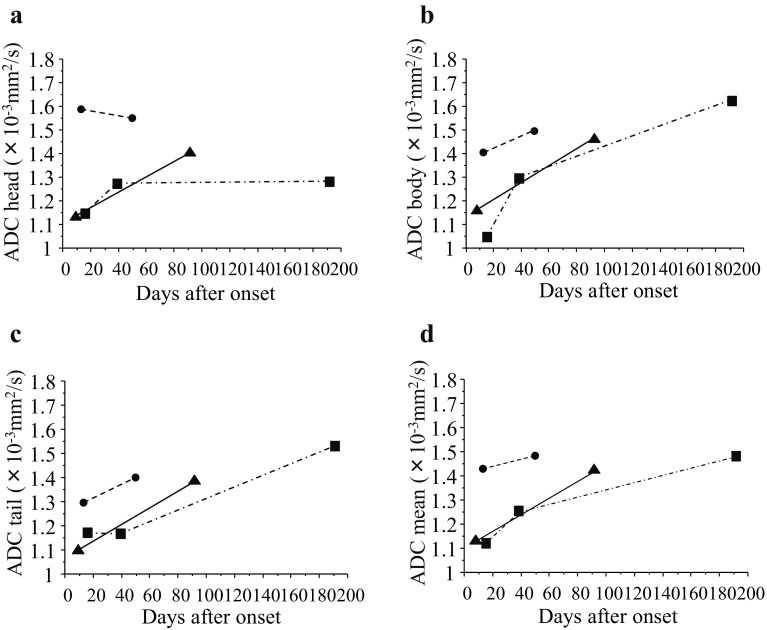
Among 14 patients with fulminant type 1 diabetes, the chronological changes in the ADC values were able to be observed up to 200 days in 3 patients (Fig. 4). ADC values tended to increase with a lapse in time.
Fig. 4.
Chronological changes in ADC values in each part of the head (a), body (b), tail (c), and mean (d) of the pancreas in patients with fulminant type 1 diabetes after the onset of disease and the initiation of insulin treatment. Boxes, circles, and triangles indicate individual patients
Discussion
We have reported significant differences in ADC values of the pancreas between patients with fulminant type 1 diabetes and non-diabetic control subjects in the present study. MRI may provide an additional tool of efficient diagnosing the disease which requires immediate treatment because of its severity. Massive cellular infiltration consisting of T cells and macrophages was detected in both endocrine and exocrine pancreas a few days after the onset of fulminant type 1 diabetes [23, 24]. It might cause the low ADC values through the restriction of water diffusion.
We aimed to clarify the importance of DWI in this study because the existing indices, including plasma glucose and HbA1c, are not satisfactorily specific to diagnose fulminant type 1 diabetes [7]. The present study revealed that ADC values could diagnose fulminant type 1 diabetes with moderate accuracy. All ADC values of the pancreatic head, body and tail showed high sensitivity and specificity, and the mean ADC values were the most efficient for diagnosing fulminant type 1 diabetes. The best cut-off value indicated that the sensitivity was 86% and the specificity was 71% using DWI. ADC values were also efficient for two marginal patients with fulminant type 1 diabetes; one with lacked definite elevation in exocrine pancreatic enzymes and another with higher HbA1c levels than the criterion because of previously diagnosed glucose intolerance.
We demonstrated that ADC values of the pancreas were negatively correlated with plasma glucose at disease onset and positively with arterial pH. It is known that massive cell infiltration into the pancreas leads to beta-cell destruction followed by absolute insulin deficiency in fulminant type 1 diabetes. Absolute insulin deficiency results in hyperglycemia, acceleration of lipolysis in the adipose tissue, and finally in the increment of ketogenesis in the liver. This might indicate that low ADC values reflect severe metabolic disorders. Recent studies have shown that DWI can be used to detect tumor lesions as well as the assessment of severity of other diseases. ADC values have been reported as having an association with the activity index of Crohn’s disease [12], degree of pancreatic fibrosis [25], and pathology scores of chronic kidney disease [26]. Although patients with fulminant type 1 diabetes were significantly younger than non-diabetic control subjects in this study, there was no correlation between pancreatic ADC values and age in a previous report [27].
In the present study, serum amylase levels did not correlate with ADC values. Thus, the value might parallel the degree of cellular infiltration, but not exocrine disorder as shown by increased amylase levels in fulminant type 1 diabetes in this study.
We confirmed that the pancreatic ADC values of three patients with fulminant type 1 diabetes increased in a course of several months after the first MRI image at the disease onset. There are several reports showing that ADC values changed by treatment over a few months. Taniguchi et al. showed in patients with autoimmune pancreatitis that ADC values of the pancreas were lower than 1.0 × 10−3 mm2/s before treatment, but they increased to 1.3–1.5 × 10−3 mm2/s after steroid therapy [16]. In addition, ADC values were also shown to have increased after therapy for cervical cancer or lymphoma [28, 29].
This is a retrospective study that has a limitation. The number of patients with fulminant type 1 diabetes in whom abdominal MRI test was performed at the onset of disease is limited. To minimize the variability of ADC values, we selected 3 vendors, Siemens Medical Solutions, GE Healthcare, Philips Healthcare, since ADC values obtained with these 3 vendors have been shown comparable each other [22]. Despite the small number of patients, application of these 3 vendors enabled us to demonstrate significant difference of ADC values between patients with fulminant type 1 diabetes and non-diabetic control subjects with simple imaging parameters. It is also a limitation that we could not make a comparison of ADC values between fulminant type 1 diabetes and other type 1 or type 2 diabetes in this retrospective study. A prospective study is required to confirm our present results.
In conclusion, the ADC values of pancreas were significantly lower in patients with fulminant type 1 diabetes than in controls. Low ADC values may reflect the severity of metabolic disorders in patients with fulminant type 1 diabetes. DWI may be an additional tool for making an efficient diagnosis of fulminant type 1 diabetes including patients difficult to diagnose.
Acknowledgements
The authors thank another member of the Japan Diabetes Society Committee on Fulminant Type 1 Diabetes Mellitus Research, Seiho Nagafuchi (Department of Medical Science and Technology, Graduate School of Medical Sciences, Kyushu University) for discussion. Japan Diabetes Society Committee on Fulminant Type 1 Diabetes Mellitus Research: Akihisa Imagawa, Norio Abiru, Takuya Awata, Hiroshi Ikegami, Yasuko Uchigata, Yoichi Oikawa, Haruhiko Osawa, Hiroshi Kajio, Eiji Kawasaki, Yumiko Kawabata, Junji Kozawa, Akira Shimada, Kazuma Takahashi, Shoichiro Tanaka, Daisuke Chujo, Tomoyasu Fukui, Junnosuke Miura, Kazuki Yasuda, Hisafumi Yasuda, Tetsuro Kobayashi, Toshiaki Hanafusa.
Conflict of interest
The authors declare no conflicts of interest.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Opt-out opportunities are provided to study subjects.
Footnotes
Members of the Japan Diabetes Society Committee on Fulminant Type 1 Diabetes Mellitus Research are listed in acknowledgements.
Contributor Information
Akihisa Imagawa, Phone: +81-72-683-1221, Email: imagawa@osaka-med.ac.jp.
For the consultation of Japan Diabetes Society Committee on Fulminant Type 1 Diabetes Mellitus Research:
Akihisa Imagawa, Norio Abiru, Takuya Awata, Hiroshi Ikegami, Yasuko Uchigata, Yoichi Oikawa, Haruhiko Osawa, Hiroshi Kajio, Eiji Kawasaki, Yumiko Kawabata, Junji Kozawa, Akira Shimada, Kazuma Takahashi, Shoichiro Tanaka, Daisuke Chujo, Tomoyasu Fukui, Junnosuke Miura, Kazuki Yasuda, Hisafumi Yasuda, Tetsuro Kobayashi, and Toshiaki Hanafusa
References
- 1.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Cho YM, Kim JT, Ko KS, et al. Fulminant type 1 diabetes in Korea: high prevalence among patients with adult-onset type 1 diabetes. Diabetologia. 2007;50:2276–2279. doi: 10.1007/s00125-007-0812-z. [DOI] [PubMed] [Google Scholar]
- 4.Luo S, Zhang Z, Li X, et al. Fulminant type 1 diabetes: a collaborative clinical cases investigation in China. Acta Diabetol. 2013;50:53–59. doi: 10.1007/s00592-011-0362-1. [DOI] [PubMed] [Google Scholar]
- 5.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y, For the Osaka IDDM Study Group A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 6.Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26:2345–2352. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 7.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3:36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka S, Kobayashi T, Momotsu T. A novel subtype of type 1 diabetes mellitus. N Engl J Med. 2000;342:1835–1837. doi: 10.1056/NEJM200006153422413. [DOI] [PubMed] [Google Scholar]
- 9.Seitz RJ, Meisel S, Weller P, Junghans U, Wittsack HJ, Siebler M. Initial ischemic event: perfusion-weighted MR imaging and apparent diffusion coefficient for stroke evolution. Radiology. 2005;237:1020–1028. doi: 10.1148/radiol.2373041435. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–991. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 11.Taouli B, Tolia AJ, Losada M, et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 12.Hordonneau C, Buisson A, Scanzi J, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol. 2014;109:89–98. doi: 10.1038/ajg.2013.385. [DOI] [PubMed] [Google Scholar]
- 13.Shinya S, Sasaki T, Nakagawa Y, et al. The efficacy of diffusion-weighted imaging for the detection and evaluation of acute pancreatitis. Hepatogastroenterology. 2009;56:1407–1410. [PubMed] [Google Scholar]
- 14.Thomas S, Kayhan A, Lakadamyali H, et al. Diffusion MRI of acute pancreatitis and comparison with normal individuals using ADC values. Emerg Radiol. 2012;19:5–9. doi: 10.1007/s10140-011-0983-2. [DOI] [PubMed] [Google Scholar]
- 15.Sandrasegaran K, Nutakki K, Tahir B, et al. Use of diffusion-weighted MRI to differentiate chronic pancreatitis from pancreatic cancer. Am J Roentgenol. 2013;201:1002–1008. doi: 10.2214/AJR.12.10170. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi T, Kobayashi H, Nishikawa K, et al. Diffusion-weighted magnetic resonance imaging in autoimmune pancreatitis. Jpn J Radiol. 2009;27:138–142. doi: 10.1007/s11604-008-0311-2. [DOI] [PubMed] [Google Scholar]
- 17.Kamisawa T, Takuma K, Anjiki H, et al. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010;105:1870–1875. doi: 10.1038/ajg.2010.87. [DOI] [PubMed] [Google Scholar]
- 18.Akisik MF, Aisen AM, Sandrasegaran K, et al. Assessment of chronic pancreatitis: utility of diffusion-weighted MR imaging with secretin enhancement. Radiology. 2009;250:103–109. doi: 10.1148/radiol.2493080160. [DOI] [PubMed] [Google Scholar]
- 19.Fattahi R, Balci NC, Perman WH, et al. Pancreatic diffusion-weighted imaging (DWI): comparison between mass-forming focal pancreatitis (FP), pancreatic cancer (PC), and normal pancreas. J Magn Reson Imaging. 2009;29:350–356. doi: 10.1002/jmri.21651. [DOI] [PubMed] [Google Scholar]
- 20.Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012) J Diabetes Investig. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki Makoto, Yamada Kei, Watanabe Yoshiyuki, Matsui Mieko, Ida Masahiro, Fujiwara Shunrou, Shibata Eri. Variability in Absolute Apparent Diffusion Coefficient Values across Different Platforms May Be Substantial: A Multivendor, Multi-institutional Comparison Study. Radiology. 2008;249(2):624–630. doi: 10.1148/radiol.2492071681. [DOI] [PubMed] [Google Scholar]
- 22.Donati OF, Chong D, Nanz D, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014;270:454–463. doi: 10.1148/radiol.13130819. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Nishida Y, Aida K, et al. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes. 2009;58:2285–2291. doi: 10.2337/db09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibasaki S, Imagawa A, Tauriainen S, et al. Expression of toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–219. doi: 10.1507/endocrj.K09E-291. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe H, Kanematsu M, Tanaka K, et al. Fibrosis and postoperative fistula of the pancreas: correlation with MR imaging findings—preliminary results. Radiology. 2014;270:791–799. doi: 10.1148/radiol.13131194. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Li J, Zhang L, Chen Y, Zhang M, Yan F. Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol. 2014;83:756–762. doi: 10.1016/j.ejrad.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Ma C, Pan CS, Zhang HG, et al. Diffusion-weighted MRI of the normal adult pancreas: the effect of age on apparent diffusion coefficient values. Clin Radiol. 2013;68:e532–e537. doi: 10.1016/j.crad.2013.05.100. [DOI] [PubMed] [Google Scholar]
- 28.Park JJ, Kim CK, Park SY, et al. Assessment of early response to concurrent chemoradiotherapy in cervical cancer: value of diffusion-weighted and dynamic contrast-enhanced MR imaging. Magn Reson Imaging. 2014;32:993–1000. doi: 10.1016/j.mri.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Horger M, Claussen C, Kramer U, Fenchel M, Lichy M, Kaufmann S. Very early indicators of response to systemic therapy in lymphoma patients based on alterations in water diffusivity—a preliminary experience in 20 patients undergoing whole-body diffusion-weighted imaging. Eur J Radiol. 2014;83:1655–1664. doi: 10.1016/j.ejrad.2014.05.027. [DOI] [PubMed] [Google Scholar]