Abstract
Abstract
A number of studies have reported the usefulness of monitoring skin temperature at local points in reducing the risk of ulceration. Thermography has the advantage of being able to visualize morphological temperature distribution. We reported that inflammation was detected by thermography in 10% of diabetes mellitus (DM) patients with foot calluses, and the area in which increased skin temperature was observed was limited to the callus. However, no reports have described thermographic findings of calluses deteriorating into foot ulcers. We report a case monitoring the skin temperature distribution using thermography, which might be useful for predicting ulceration.
Case
A 53-year-old male patient, diagnosed with type 2 DM, was treated with insulin therapy. The duration of DM was 4 years. He was also diagnosed with dyslipidemia and hypertension. Using thermography, the skin temperature was evaluated in the patient with calluses on the 5th metatarsal heads. Areas of increased skin temperature were observed, involving not only the callused part, but also the plantar arch. We shaved his calluses once a month and explained the importance of his therapeutic shoes to prevent the ulcers. After 43 months, an ulcer developed.
Discussions
Thermographic findings of an extended area of increased skin temperature not limited to the callus may suggest the progression of a callus to ulcer. Expansion of the area of increased skin temperature might show the inflammation or infection extending along the fascia. Based on these findings, thermography could provide a useful assessment of callus in DM patients with a high risk of progression.
Keywords: Thermography, Diabetes mellitus, Callus, Foot ulcer
Introduction
Once diabetic foot ulcer develops, the physical condition, long-term prognosis [1, 2], and quality of life of the patient are severely affected [3, 4]. The establishment of effective preventive methods for diabetic foot ulcers is therefore an urgent issue, since the number of patients with diabetes mellitus (DM) is increasing [5].
A number of studies have reported the usefulness of monitoring skin temperature at local points in reducing the risk of ulceration [6–8]. However, compared with conventional devices for measuring skin temperature at local points, such as contact infrared skin thermometers, thermography has the advantage of being able to visualize temperature distribution morphologically [9–13]. We reported that latent inflammation was detected by thermography in 10% of subjects with diabetes in a cross-sectional study of subjects with foot calluses [9]. An increased area of skin temperature limited to the region of the callus was observed in that study. However, no reports have described thermographic findings of calluses deteriorating into foot ulcers. We report a case monitoring the skin temperature distribution using thermography, which might be useful for predicting ulceration. This study was approved by the ethics committee at the Graduate School of Medicine and Faculty of Medicine, University of Tokyo (no. 3078-(2)).
Case
A 53-year-old male patient, diagnosed with type 2 DM, was treated with insulin therapy. The duration of DM was 4 years, and his HbA1c was 6.9%. He was also diagnosed with dyslipidemia and hypertension. He had normal sensation for the vibration test and 5.07 monofilament test, but he had a reduced Achilles tendon reflex, abnormal coefficient of variation of R-R intervals (0.58%), and abnormal feeling of the feet. He had no angiopathy [ankle brachial pressure index, 1.17 (right) and 1.11 (left)]. Stage 2 diabetic nephropathy was identified. No data were available regarding diabetic retinopathy.
Present history
He visited the DM foot clinic at the University of Tokyo Hospital, Japan, once a month for treatment of the calluses located on the fifth metatarsal heads of both feet. Forefoot pes varus and pes cavus were present, which might have influenced formation of the calluses. The healthcare provider proposed several times that the patient have therapeutic shoes made to reduce plantar pressure. He agreed to the creation of therapeutic shoes, but did not visit a prosthetist because he was busy. Fourteen months after the first visit to the foot clinic, he had therapeutic shoes made and visited the foot clinic wearing these shoes. However, he might not have been using the therapeutic shoes despite his insistence to the contrary, because the therapeutic shoes seemed to be new.
Seventeen months after the first visit to the DM foot clinic, the callus of the 5th metatarsal head of the right foot progressed to an ulcer, and the patient was treated in the dermatology department. His C-reactive protein (CRP) level was 0.42 mg/dl.
Nineteen months after the first visit to the foot clinic, thermography of both feet was performed using a Thermotracer TH5108ME (NEC Avio, Tokyo, Japan) (Fig. 1). Thermographic images after 15 min of equilibration were used to evaluate skin temperature. We adjusted temperature intervals in the thermographs to 1.5 °C using NS9200 software (NEC Avio) to perform qualitative evaluation of relative differences in temperatures at the site. The area of increased skin temperature involved not only the area of ulceration, but also internally in the right foot. On the other hand, the area of increased skin temperature was limited to the callus in the left foot. The CRP level was 0.11 mg/dl. We shaved his calluses once a month. We also continued to advise wearing therapeutic shoes because his therapeutic shoes seemed to be new. The ulcer of the right foot reached a maximum size of 6–7 mm and became epithelialized by 36 months after the first visit to the foot clinic.
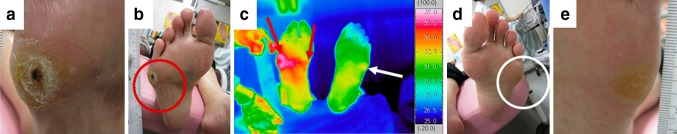
Fig. 1.
Thermal and visual images after 17 months. a Appearance of the ulcer on the right foot. b Appearance of the right sole (red circle indicates the ulcer). c Thermal image of both feet. In addition to the ulcerous region, increased skin temperature was also observed internally on the right foot (red arrows), although this was limited to the callused area on the left foot (white arrow). d Appearance of the left sole (white circle indicates the callus). e Appearance of the callus on the left foot
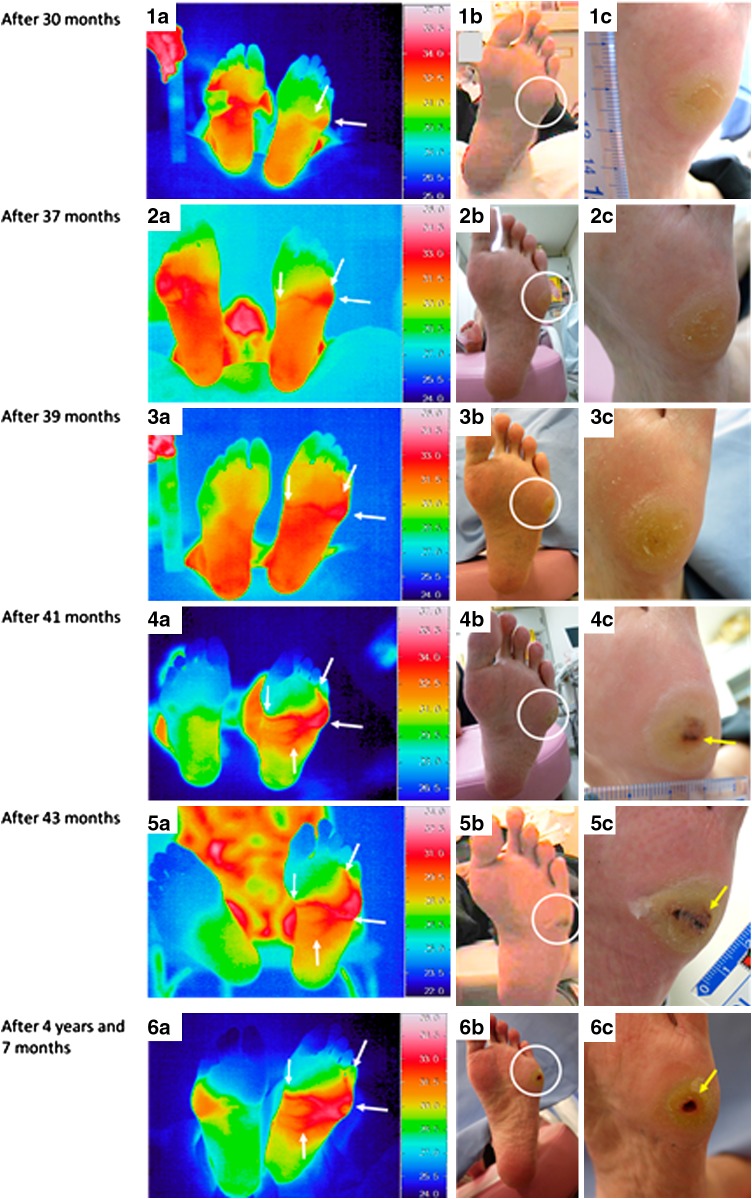
Thermographic images of the feet were again taken using a Thermotracer TH7800 N (NEC Avio) at 30, 37 and 39 months after the first visit to the foot clinic (Fig. 2 1a–3c). The area of increased skin temperature extended from the callus part to internally in the left foot at 30 months, although internal hemorrhage within the callus was not observed. Furthermore, the area of increased skin temperature extending internally in the left foot remained at 37 and 39 months. CRP level was 0.06, 0.11 and 0.29 mg/dl, respectively. We continued to shave his calluses once a month and explain importance of his therapeutic shoes to prevent the ulcer to him.
Fig. 2.
Thermal and visual images. 1a Thermal image of both soles. The area of increased skin temperature extended from the callused part to internally on the left foot (white arrows). The right foot was covered with dressing. 1b Visual image of the left sole (white circle indicates the callus). 1c Visual image of the callus on the left foot. Internal hemorrhage in the callus was not observed. 2a Thermal image of both soles. The increased skin temperature still extends internally on the left foot (white arrows). The right foot was covered with dressing. 2b Appearance of the left sole (white circle indicates the callus). 2c Appearance of the callus on the left foot. Internal hemorrhage in the callus was not observed. 3a Thermal image of both soles. The elevated skin temperature that extends internally continues in the left foot (white arrows). 3b Visual image of the left sole (white circle indicates the callus). 3c Visual image of the callus on the left foot. Internal hemorrhage in the callus was not observed. 4a Thermal image of both soles. The increased skin temperature extending internally continues on the left foot (white arrows). 4b Appearance of the left sole (white circle indicates the callus). 4c Appearance of the callus on the left foot. Internal hemorrhage within the callus is observed in the left foot (yellow arrow). 5a Thermal image of both soles. Increased skin temperature extending internally on the left foot (white arrows). 5b Visual image of the left sole (white circle indicates the callus). 5c Appearance of the callus on the left foot. Internal bleeding continues in the left foot (yellow arrow). 6a Thermal image of both soles. Increased skin temperature extending internally on the left foot (white arrows). 6b Appearance of the left sole (white circle indicates the ulcer). 6c Appearance of the ulcer on the left foot (yellow arrow)
At 41 months after the first visit, the area of increased skin temperature extending internally continued, and internal hemorrhage within the callus in the left foot was still apparent (Fig. 2 4a–c). The CRP level was 0.15 mg/dl.
After 43 months, because both increased skin temperature extending internally and internal bleeding continued, we consulted the department of dermatology, and diabetic foot ulcer in the left foot was diagnosed (Fig. 2 5a–c). CRP level was 0.16 mg/dl. At this time, abnormal findings were discovered for the first time with the 5.07 monofilament test, although no abnormalities were found in examinations for angiopathy.
At 4 years and 7 months after the first visit, diabetic foot ulcer recurred in the left foot. The area of increased skin temperature also extended from the callused part to the plantar arch on the left foot (Fig. 2 6a–c).
Discussion
To the best of our knowledge, this represents the first report of thermography in a subject with diabetic foot ulcer due to callus deterioration. Notably, the area of increased skin temperature that involved not only the callus, but also internally within the foot had been observed before hemorrhage within the callus appeared. In our previous study regarding thermographic findings of callus with inflammation, the area in which increased skin temperature was observed was limited to the callus [9]. Thermographic findings of an area of increased skin temperature extending beyond the callus might thus suggest the possibility of callus progression to ulcer.
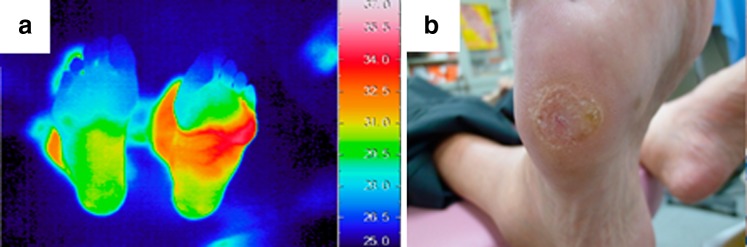
The importance of an increased skin temperature is that heat is one of the signs of inflammation. Neither swelling nor redness as alternative signs of inflammation was observed macroscopically, presumably because the thick stratum corneum on the sole might mask such signs. Regarding the time course of thermographic findings of the right foot after the recovery of foot ulcer, the thermographic finding of an area of increased skin temperature extending beyond the callus or ulcer resolved as the foot ulcer improved (Fig. 3). This result might support our hypothesis of which the thermographic finding of an area of increased skin temperature extending beyond the callus is associated with the subclinical inflammation which may progress to the clinical foot ulcer. In previous study, we found that an area of increased skin temperature extending to the ankle can be a sign of osteomyelitis [12, 13]. Expansion of the area of increased skin temperature might show the inflammation or infection extending along the fascia. More detailed analyses are needed to confirm whether extension of increased skin temperature is a finding specific to callus on the cusp of progressing to ulcer. In addition, callus in other regions might show different findings. When thermographic findings of diabetic foot ulcer due to callus deterioration are identified on further studies, thermography might be useful for patient education. We could not explain to this patient that the risk of ulceration was high. This was because the thermographic findings of diabetic foot ulcer due to callus deterioration were unclear even if skin temperature could be explained to be rising because of inflammation. The patient therefore did not recognize that the risk of ulceration was high and did not consistently use the therapeutic shoes provided.
Fig. 3.
Thermal and visual images after 41 months. a Thermal image of both feet. Regarding the right foot, the thermographic finding of an area of increased skin temperature extending beyond the callus or ulcer resolved as the foot ulcer improved. b Visual image of the callus on the right foot. The foot ulcer was improved
It is considered that angiopathy or cellulitis can influence the skin temperature. It is known that the skin temperature of patients with ischemia is low. Furthermore, skin temperature might be insensitive to inflammation because of insufficient blood supply. The area of increased skin temperature due to extensive cellulitis might mask the thermographic finding of an area of increased skin temperature extending beyond the callus. The relationship between plantar pressure and skin temperature was unclear in this case, although temperature has recently been reported as a surrogate for shear [14]. However, the relationship might have been weak in this case, because thermographic images were taken after 15 min of equilibration and the area of increased skin temperature extended to the plantar arch, which is an unloaded site. An ulcer should be suspected when internal bleeding in a callus is observed. However, the callus was not diagnosed as an ulcer at 41 months after the first visit, and osteomyelitis was not checked. Therefore, it was not able to indicate a relation between osteomyelitis and skin temperature in this case.
Thermography was used to visualize temperature distributions in this report, and we found that the area of increased skin temperature extended beyond the callus in a subject with diabetic foot ulcer due to callus deterioration. This type of skin temperature evaluation may only be possible with thermography, which might therefore prove useful for assessing the risk of callus progressing to ulcer in DM patients.
Conflict of interest
T. Yamauchi received a scholarship Grant from Sanofi. M. Oe, K. Takehara, H. Noguchi, Y. Ohashi, A. Amemiya, H. Sakoda, R. Suzuki, K. Ueki, T. Kadowaki, and H. Sanada declare that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from the patient for being included in the study.
References
- 1.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV. Diabetic foot disorders. A clinical practice guideline (2006 revision) J Foot Ankle Surg. 2006;45(5):S1–S66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Carter EA, Lindsay R, Henly SJ, Ness FK, Welty TK, Lee ET, Howard BV. Relation of lower-extremity amputation to all-cause and cardiovascular disease mortality in American Indians: the Strong Heart Study. Diabetes Care. 2004;27(6):1286–1293. doi: 10.2337/diacare.27.6.1286. [DOI] [PubMed] [Google Scholar]
- 3.Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complicat. 2000;14:235–241. doi: 10.1016/S1056-8727(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 4.Nabuurs-Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia. 2005;48:1906–1910. doi: 10.1007/s00125-005-1856-6. [DOI] [PubMed] [Google Scholar]
- 5.International diabetes federation. The IDF diabetes Atlas (the 7th edition). 2015.
- 6.Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, Armstrong DG, Agrawal CM. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14–22. doi: 10.2337/dc06-1600. [DOI] [PubMed] [Google Scholar]
- 8.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 9.Nishide K, Nagase T, Oba M, Oe M, Ohashi Y, Iizaka S, Nakagami G, Kadowaki T, Sanada H. Ultrasonographic and thermographic screening for latent inflammation in diabetic foot callus. Diabetes Res Clin Pract. 2009;85(3):304–309. doi: 10.1016/j.diabres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Roback K, Johansson M, Starkhammar A. Feasibility of a thermographic method for early detection of foot disorders in diabetes. Diabetes Technol Ter. 2009;11(10):663–667. doi: 10.1089/dia.2009.0053. [DOI] [PubMed] [Google Scholar]
- 11.Nagase T, Sanada H, Takehara K, Oe M, Iizaka S, Ohashi Y, Oba M, Kadowaki T, Nakagami G. Variations of plantar thermographic patterns in normal controls and non-ulcer diabetic patients: novel classification using angiosome concept. J Plast Reconstr Aesthet Surg. 2011;64(7):860–866. doi: 10.1016/j.bjps.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Oe M, Yotsu RR, Sanada H, Nagase T, Tamaki T. Thermographic findings in a case of type 2 diabetes with foot ulcer and osteomyelitis. J Wound Care. 2012;21(6):274–278. doi: 10.12968/jowc.2012.21.6.274. [DOI] [PubMed] [Google Scholar]
- 13.Oe M, Yotsu R, Sanada H, Nagase T, Tamaki T. Screening for osteomyelitis using thermography in patients with diabetic foot. Ulcers. 2013. doi:10.1155/2013/284294.
- 14.Yavuz M, Brem RW, Davis BL, Patel J, Osbourne A, Matassini MR, Wood DA, Nwokolo IO. Temperature as a predictive tool for plantar triaxial loading. J Biomech. 2014;47(15):3767–3770. doi: 10.1016/j.jbiomech.2014.09.028. [DOI] [PubMed] [Google Scholar]