Abstract
Metabolic processes in different tissues and remote organs are under coordinated systemic regulation, allowing adaptation to a variety of external circumstances. Neuronal signals as well as humoral factors, such as nutrients, growth factors, and hormones, have attracted increasing attention for their roles in this interorgan metabolic network, responsible for the maintenance of metabolic homeostasis at the whole-body level. These interorgan communications within an organism are considered to be diverse and, in fact, we identified previously unknown neuronal relay systems originating in the liver which modulate energy, glucose, and lipid metabolism. Furthermore, when nutrient overload is prolonged, these neuronal mechanisms, which function as an endogenous defense system against obesity development, contribute to the pathophysiological states of metabolic syndrome characterized by obesity-associated features. Therefore, these interorgan neuronal systems are considered to be possible molecular targets for treating metabolic syndrome. We herein review the precise mechanisms underlying the functions of the mammalian interorgan neuronal network, especially the pathways from the liver to several other organs, focusing on their significance and roles in the development of metabolic syndrome.
Keywords: Interorgan neuronal network, Metabolic syndrome, Hypertension, Hypertriglyceridemia, PPARγ, Amino acids, mTOR
Introduction
Currently, the worldwide prevalence of metabolic syndrome is increasing at an alarming rate, which is a reflection of the westernization of dietary habits and altered food preferences [1, 2]. Metabolic syndrome represents a collection of pathological states based on obesity and obesity-associated pathogenesis, including hypertension, glucose intolerance, and dyslipidemia. The combination of these conditions accelerates the risk for cardiovascular and cerebrovascular morbidities. In addition, the pathophysiological bases of metabolic syndrome stem from genetic or environmental factors, possibly in combination, such that further insights into the mechanisms underlying the pathogenesis of metabolic syndrome are eagerly awaited [3].
Communication and coordination among various organs are essential for maintaining the homeostasis of systemic metabolic processes at the whole-body level [4, 5]. In addition, metabolic processes in these organs are regulated in a coordinated fashion by both humoral and neuronal factors. The autonomic nervous system is increasingly being recognized as an important component of this interorgan metabolic network and has attracted considerable research attention [3, 6]. Conducting research under current circumstances, we identified interorgan neuronal networks originating in the liver, and elucidated the physiological roles of these systems in regulating several metabolic processes at the whole-body level [7].
However, nutrient overloads due to excess energy intake, a hallmark of obesity development, may affect these interorgan systems which regulate metabolism, resulting in the progressive worsening of metabolic dysregulation [3]. We have also demonstrated that these neuronal systems, ironically, contribute to the development of metabolic syndrome features, such as weight gain [8], hypertension [9], and hyperlipidemia [10]. In other words, our results indicate that the newly identified interorgan neuronal networks have pathophysiological roles in the development of metabolic syndrome, raising the possibility of them serving as novel therapeutic targets for metabolic syndrome.
Mechanisms mediated by the interorgan neuronal network
Metabolic processes at the whole-body level are intricately regulated via interactions among remote organs. These interorgan communications are considered to play fundamental roles in maintaining metabolic homeostasis. Metabolic processes within organs are communicated to other organs via the neuronal network in addition to humoral factors, including insulin, adipokines [11, 12], hepatokines [13, 14], and myokines [15, 16]. These metabolic communications are also referred to as the “Metabolic Information Highway,” which has become an important area of research worldwide.
We have identified a neuronal relay system originating in white adipose tissue (WAT) which functions by modulating UCP1 expression in WAT. This neuronal system is considered to ameliorate the deterioration of glucose metabolism and the state of leptin resistance in states of obesity, via afferent sympathetic nerves traveling from WAT to the brain [17].
Next, we identified neuronal relay systems originating in the liver which have significant roles in regulating energy balance [7, 8] and glucose metabolism [18], both of which are essential for adapting to the excess nutrition availability in our current “age of plenty.” These systems are considered to be regulated by either the afferent vagal or splanchnic nerves traveling from the liver to the brain, sending neuronal signals to adipose tissue [7, 8] or the pancreas [18], respectively.
In addition, several previous reports have indicated the importance of neuronal networks and have demonstrated that the brain plays a central role in mediating these neuronal networks [19–21]. For example, the mechanisms by which alterations of serum glucose, lipid, and leptin levels in the brain—especially the hypothalamus—are sensed, as well as those regulating hepatic glucose and lipid production via the hepatic vagal nerve, have been identified [19, 20]. These systems are considered to mainly be regulated by the efferent vagal nerve traveling from the brain to the liver. On the other hand, the conditions associated with obesity may attenuate these regulatory systems in the hypothalamus and thereby exacerbate pathophysiological states such as serum glucose and lipid elevations, contributing, respectively, to the development of diabetes and dyslipidemia [19, 20, 22].
Moreover, metabolic alterations in the duodenum and other portions of the intestinal tract reportedly regulate hepatic glucose production via the neuronal network, an interactive process referred to as the gut–brain–liver axis. This system is considered to consist of both afferent nerves traveling from the gut to the brain and efferent vagal nerves traveling from the brain to the liver [23, 24]. Furthermore, obesity reportedly attenuates this regulatory system and thereby results in the dysregulation of hepatic gluconeogenesis, leading to serum glucose elevation, i.e., hyperglycemia [24].
Collectively, the close connections between the liver, brain, and other organs, mediated by neuronal networks functioning throughout the body, are considered to maintain metabolic homeostasis. Furthermore, prolonged obesity in the current age of plenty is speculated to lead to dysregulation of these neuronal systems, thereby exacerbating metabolic disorders.
Roles of the interorgan neuronal network originating in the liver
Interorgan communication and energy metabolism
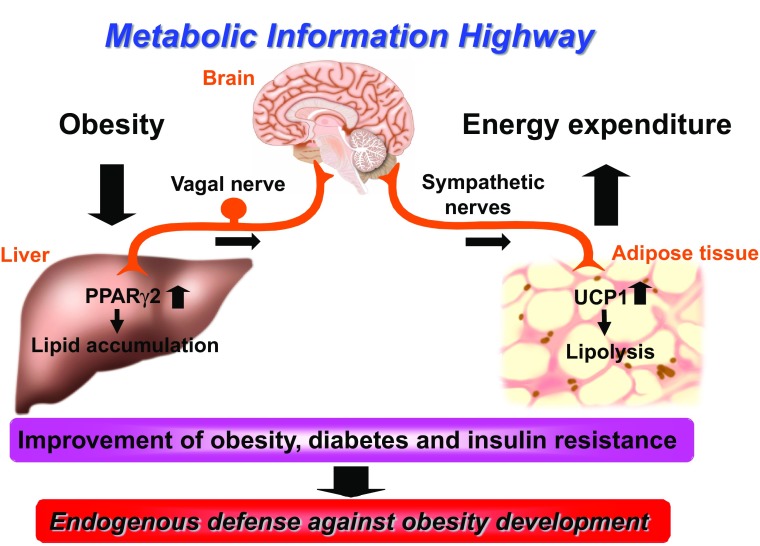
In a number of murine obesity models as well as in obese human subjects, hepatic expression of PPARγ—especially PPARγ2, a transcriptional factor which activates genes involved in lipid storage and production—has been demonstrated to be upregulated [25, 26]. Hepatic expression of PPARγ2 is considered to mimic the obesity-related phenotype of the liver, i.e., the development of fatty liver. Hepatic lipid accumulation through PPARγ2 induction modulates energy metabolism by activating the sympathetic nervous system and enhancing energy expenditure and lipolysis in adipose tissue via the neuronal network traveling from the liver to adipose tissue. This endogenous system, which is present in all mammals, appears to protect multi-organ organisms from obesity development by regulating systemic energy metabolism via sympathetic nervous system activation (Fig. 1) [7]. Excess energy intake, i.e., beyond physiological requirements, reportedly upregulates hepatic expression of glucokinase, the enzyme catalyzing the first and rate-limiting step of glycolysis [27, 28]. Hepatic expression of glucokinase is also considered to mimic the obesity-related phenotype of the liver. The glycolytic alterations in the liver resulting from increased hepatic glucokinase expression lead to decreased UCP1 expression in brown adipose tissue (BAT) via neuronal network signals from the liver to BAT. This system thereby mediates the suppression of thermogenesis in BAT, with consequent obesity development. This pathway is considered to serve as an energy-saving mechanism at the whole-body level by regulating adaptive thermogenesis via the UCP1 level in BAT [8].
Fig. 1.
Physiology of the interorgan neuronal network. The interorgan neuronal network traveling from the liver to adipose tissues regulates systemic glucose and energy metabolism. This pathway functions as an endogenous defense mechanism against obesity development under conditions of sustained nutirent overload
Interorgan communication and glucose metabolism
The endogenous extracellular regulated kinase (ERK) pathway is known to be activated in the livers of obese mammals [29]. Hepatic expression of the constitutively active form of mitogen-activated protein kinase/ERK kinase has actually been shown to upregulate the ERK pathway in the liver, leading to a state mimicking the obesity-related phenotype of the liver. In this state, activation of the hepatic ERK pathway increased the pancreatic β-cell mass and glucose-induced insulin secretion from these β cells via the neuronal network traveling from the liver to the pancreas [18]. This phenotype indicates that upregulation of the hepatic ERK pathway contributes to the increase in pancreatic β-cell mass and obesity-associated hyperinsulinemia in the state of insulin resistance associated with obesity development. Furthermore, we anticipate that the ability to modulate this neuronal relay, originating in the hepatic ERK pathway, would provide opportunities to promote the insulin secretion necessary to compensate for decreased insulin levels. New therapeutic targets for managing diabetes might thus arise from research on this neuronal relay.
Interorgan communication and hypertension
Humoral mechanisms, including insulin and leptin, have been proposed to be involved in the development of obesity-related disorders. For example, hyperleptinemia is also associated with blood pressure (BP) elevation and renal sympathetic activation in human subjects [30]. This is due mainly to selective leptin resistance, in which the sympathoexcitatory effect of leptin is preserved, despite resistance to the anorexigenic effect of leptin [31].
In addition to the roles of these humoral factors, numerous studies have shown sympathetic tonus to be increased in obese subjects [32], especially those with visceral adiposity [33]. Obese states, even when not accompanied by BP elevation, are considered to represent adrenergic overdrive. Furthermore, several pathological states combining to produce the metabolic syndrome have been shown to be associated with increased adrenergic drive [34]. Such activation of sympathetic tonus is considered to increase cardiac output, renal sodium absorption, and vasoconstriction, as well as potentiating the renin–angiotensin–aldosterone system, leading to the development of hypertension [35].
Sympathetic, especially afferent, nerves from the aorta and abdominal viscera reportedly play important roles in BP regulation [36]. In addition to the actions of sympathetic afferents, intraperitoneal dexamethasone administration has also been shown to raise BP via a mechanism involving hepatic PPARα and vagal afferents from the liver [37]. Thus, neuronal signals, mediated by the afferent component of the vagal nerve from the liver, are considered to be involved in determining BP in various settings.
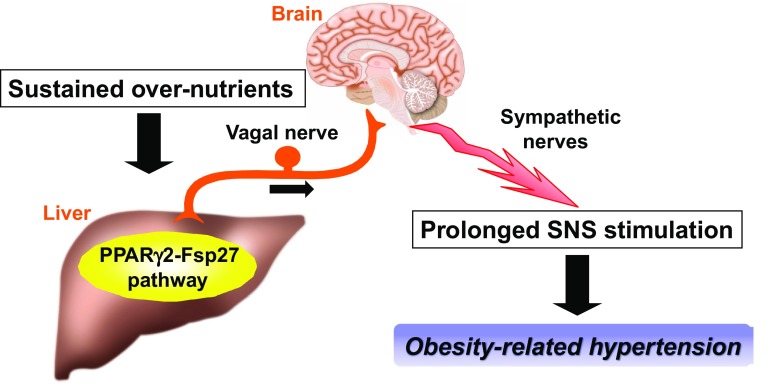
Hepatic activation of the PPARγ2-Fsp27 pathway, upregulated in the livers of obese humans and animal models, raises sympathetic tonus and thereby elevates BP. These phenotypes were inhibited by hepatic vagal nerve blockade achieved by hepatic vagotomy and capsaicin application to the hepatic vagal nerve. In addition, hepatic PPARγ2 expression in β-less mice had no effect on BP regulation. These results demonstrated that the hepatic PPARγ2-Fsp27 pathway regulates the sympathetic nervous system and controls BP via the neuronal pathway consisting of the hepatic afferent vagal nerve and sympathetic (β-adrenergic) nerves [9]. Conversely, suppression of the hepatic PPARγ2-Fsp27 pathway in obese models, by administering PPARγ2 and Fsp27 shRNAi adenoviruses, improves obesity-related hypertension. Therefore, under conditions of sustained energy overload, this defensive system against obesity, ironically, has a pathophysiological role in persistent sympathetic nervous system activation, i.e., obesity-related hypertension, a feature of the metabolic syndrome (Fig. 2) [9].
Fig. 2.
Scheme of the pathophysiology of metabolic syndrome, i.e., obesity-related hypertension. Under the conditions of sustained energy overload often present during obesity development, the interorgan neuronal network, ironically, persistently activates the sympathetic nervous system, leading to pathological states such as obesity-related hypertension
Roles of the interorgan neuronal and internutrient network originating in the liver
Amino acid metabolism and the mTOR pathway in the liver
Next, we focused on the roles of hepatic amino acid (AA) signaling in whole-body metabolism. Circulating AAs, mainly from dietary protein, are considered to enter the cytoplasm via AA transporters (AATs), which are responsible for the first step in AA signaling [38]. Recently, it has been recognized that circulating AAs as well as the AA contents of a variety of organs are increased in obesity [39]. The mammalian target of rapamycin (mTOR) pathway is known to function as a downstream target of insulin and nutrients, including AAs, and growth hormones, leading to protein synthesis and autophagy pathways that maintain the organism in a healthy state [40–42]. In addition, obese models show upregulation of the mTOR/p70-S6 kinase (S6K) pathway in several organs, including the liver [43, 44]. Therefore, hepatic expression of an AAT (SNAT2, slc38a2), which is an upstream target and the first step in the AA signaling pathway [38, 45], is considered to activate the AA/mTOR/S6K pathway in the liver [10]. Modulation of the hepatic AA/mTOR pathway introduced by SNAT2 expression is thus considered to mimic the hepatic phenotype observed in obesity.
mTOR pathway in the liver and lipid metabolism
Hepatic SNAT2 expression actually activated the hepatic AA/mTOR pathway, mimicking the obesity-related phenotype of the liver. In addition, hepatic expression of RAS homolog enriched in brain (Rheb), an activator of the mTOR pathway independent of the AA signaling pathway [46], also activated the AA/mTOR pathway in the liver. Under these conditions, hepatic activation of the mTOR pathway by SNAT2 and Rheb inductions markedly increased serum triglyceride (TG) levels, including chylomicron and very low density lipoprotein (VLDL) fractions, especially in postprandial states.
Previous studies demonstrated that the hepatic mTOR pathway is associated with lipid metabolism through fatty acid biosynthesis [47] and autophagy-regulated lipid metabolism [48]. Therefore, the mTOR pathway in the liver is considered to function as an energy sensor, modulating cellular processes involved in lipid metabolism [49] as well as systemic processes at the whole-body level.
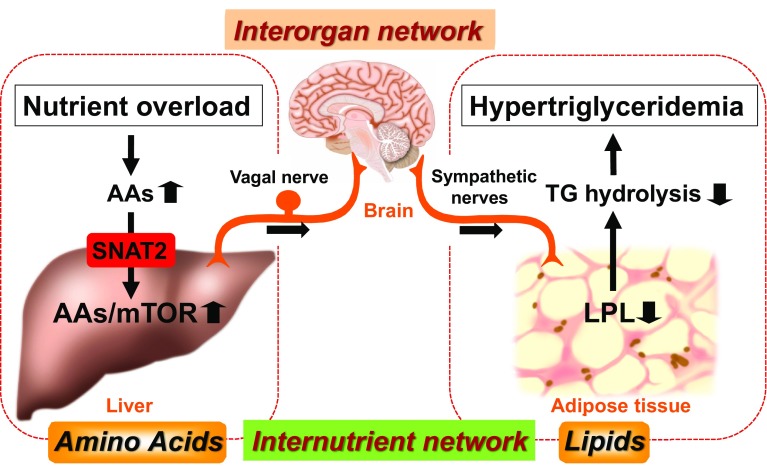
The hypertriglyceridemia observed in these models of hepatic SNAT2 and Rheb inductions is due mainly to decreased plasma TG-hydrolysis activity. The decrease in plasma TG-hydrolysis activity resulted mainly from the downregulation of adipose lipoprotein lipase (LPL) expression. On the other hand, hepatic expression of the dominant-negative form of S6K (DN-S6K) inhibited the hypertriglyceridemia observed in SNAT2 mice, indicating involvement of the hepatic mTOR/S6K pathway in regulating serum TG levels [10]. Furthermore, hepatic expression of DN-S6K in obese mice inhibited the hepatic mTOR pathway and obesity-related hypertriglyceridemia with upregulation of adipose LPL expression. Based on these results, the mTOR/S6 K pathway in the liver is, potentially, a novel therapeutic target for obesity-related dyslipidemia (hypertriglyceridemia).
Interorgan communication and lipid metabolism
Adipose LPL expression was previously reported to be negatively regulated by the sympathetic (β-adrenergic) nervous system [50]. First, hepatic vagal nerve blockade, achieved by hepatic vagotomy and capsaicin application to the hepatic vagal nerve in Rheb mice, inhibited postprandial hypertriglyceridemia and suppressed the down-regulation of adipose LPL expression. In addition, blockade of β-adrenergic nerves by β-blocker administration in Rheb mice also inhibited both postprandial hypertriglyceridemia and adipose LPL downregulation. Therefore, this novel mechanism is mediated by the interorgan neuronal relay originating from the hepatic AA/mTOR/S6K signaling pathway, and is responsible for regulating systemic lipid metabolism. Furthermore, this mechanism mediated by the interorgan neuronal network produces internutrient coordination (AA to lipid) at the whole-body level. Therefore, this interorgan/nutrient network is considered to contribute significantly to the development of obesity-related hypertriglyceridemia (Fig. 3) [10].
Fig. 3.
The pathophysiology of obesity-related hypertriglyceridemia, a hallmark of metabolic syndrome. The interorgan (liver to adipose) neuronal network is responsible for a novel form of internutrient (AA to lipid) coordination. Sustained overnutrition during obesity development excessively activates this regulatory system, leading to pathological states such as obesity-related hypertriglyceridemia
Contributions of the interorgan neuronal network to metabolic syndrome
The expotential increase in obesity rates worldwide is emerging as a major social problem with serious adverse consequences, including atherosclerotic morbidity [51, 52]. In particular, visceral obesity is prone to be associated with hypertension, glucose intolerance, and hyperlipidemia, collectively termed metabolic syndrome, the combination of which raises the risk for cardiovascular and cerebrovascular complications. We originally identified novel mechanisms mediated by neuronal networks originating mainly in the liver, and elucidated their physiological roles in maintaining metabolic homeostasis at the whole-body level. Furthermore, we have demonstrated that these endogenous systems, ironically, also have pathophysiological roles in the development of features of the metabolic syndrome, such as obesity-related hypertension (Fig. 2) [9] and hypertriglyceridemia (Fig. 3) [10].
Conclusion
Interorgan neuronal networks have begun to attract attention worldwide for their roles in maintaining metabolic homeostasis at the whole-body level in mammals. It is noteworthy that the mechanisms mediated by the neuronal pathways originating in the liver play physiological roles in regulating systemic glucose and energy metabolism [7]. These interorgan neuronal networks are considered to underlie the internutrient network, opening up a new field in nutrition science. Furthermore, in states of prolonged energy overload, these neuronal mechanisms contribute to the development of pathophysiological states, such as the components of metabolic syndrome [9, 10]. In conclusion, the discovery of new neuronal networks and the identification of molecular targets regulating these networks in the near future are eagerly anticipated.
Acknowledgments
Parts of this review were presented as the Lilly Award Lecture at the Japan Diabetes Society 2016, Kyoto, Japan. The author would like to thank Professors Hideki Katagiri and Yoshitomo Oka for their thoughtful guidance and longstanding support. The author would also like to thank Dr. Tetsuya Yamada for the initial lessons in graduate research, and my colleagues and collaborators for their helpful support and advice. Finally, the author gratefully acknowleges the the commitment and generosity of the Tohoku University Graduate School of Medicine, Tohoku University. The studies described were supported by the Japan Society for the Promotion of Science, a Research Fellowship for Young Scientists (grant number 19007896), and KAKENHI (grant numbers 23791011, 25860736, 15K09407).
Conflict of interest
The author has no conflicts of interest to declare.
Animal studies
Animal studies were conducted in accordance with Tohoku University institutional and national guidelines for the care and use of laboratory animals.
References
- 1.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–1545. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- 2.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 3.Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 4.Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol. 2016;64:1176–1186. doi: 10.1016/j.jhep.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Droujinine IA, Perrimon N. Defining the interorgan communication network: systemic coordination of organismal cellular processes under homeostasis and localized stress. Front Cell Infect Microbiol. 2013;3:82. [DOI] [PMC free article] [PubMed]
- 6.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 7.Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, Hasegawa Y, Gao J, Kaneko K, Iwasaki H, Ishihara H, Sasano H, Inukai K, Mizuguchi H, Asano T, Shiota M, Nakazato M, Oka Y. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- 8.Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, Oka Y, Katagiri H. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 2012;16:825–832. doi: 10.1016/j.cmet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Uno K, Yamada T, Ishigaki Y, Imai J, Hasegawa Y, Gao J, Kaneko K, Matsusue K, Yamazaki T, Oka Y, Katagiri H. Hepatic peroxisome proliferator-activated receptor-γ-fat-specific protein 27 pathway contributes to obesity-related hypertension via afferent vagal signals. Eur Heart J. 2012;33:1279–1289. doi: 10.1093/eurheartj/ehr265. [DOI] [PubMed] [Google Scholar]
- 10.Uno K, Yamada T, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Kaneko K, Ono H, Asano T, Oka Y, Katagiri H. A hepatic amino acid/mTOR/S6 K-dependent signalling pathway modulates systemic lipid metabolism via neuronal signals. Nature Commun. 2015;6:7940. [DOI] [PMC free article] [PubMed]
- 11.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014;210:733–753. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 13.Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9:144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 14.Iroz A, Couty JP, Postic C. Hepatokines: unlocking the multi-organ network in metabolic diseases. Diabetologia. 2015;58:1699–1703. doi: 10.1007/s00125-015-3634-4. [DOI] [PubMed] [Google Scholar]
- 15.Rai M, Demontis F. Systemic nutrient and stress signaling via myokines and myometabolites. Annu Rev Physiol. 2016;78:85–107. [DOI] [PubMed]
- 16.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim do H, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. [DOI] [PubMed]
- 17.Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Niijima A, Mano H, Aburatani H, Asano T, Oka Y. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 2006;3:223–229. doi: 10.1016/j.cmet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, Ishihara H, Niijima A, Nakazato M, Asano T, Minokoshi Y, Oka Y. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 19.Sandoval DA, Obici S, Seeley RJ. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discovery. 2009;8:386–398. doi: 10.1038/nrd2874. [DOI] [PubMed] [Google Scholar]
- 20.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat Med. 2010;16:392–395. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- 22.Caspi L, Wang PY, Lam TK. A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab. 2007;6:99–104. doi: 10.1016/j.cmet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 24.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, Dube GP. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem. 2001;224:29–37. doi: 10.1023/A:1011927113563. [DOI] [PubMed] [Google Scholar]
- 26.Westerbacka J, Kolak M, Kiviluoto T, Arkkila P, Siren J, Hamsten A, Fisher RM, Yki-Jarvinen H. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 27.Belfiore F, Romeo F, Iannello S, Salamone C. The glucose-6-phosphatase/glucokinase ratio in the liver of obese-diabetic subjects. Biochem Med Metab Biol. 1989;41:77–80. doi: 10.1016/0885-4505(89)90011-X. [DOI] [PubMed] [Google Scholar]
- 28.Spydevold SO, Greenbaum AL, Baquer NZ, McLean P. Adaptive responses of enzymes of carbohydrate and lipid metabolism to dietary alteration in genetically obese Zucker rats (fa/fa) Eur J Biochem/FEBS. 1978;89:329–339. doi: 10.1111/j.1432-1033.1978.tb12534.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res. 2001;61:5016–5023. [PubMed] [Google Scholar]
- 30.Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010;56:623–628. doi: 10.1161/HYPERTENSIONAHA.109.148213. [DOI] [PubMed] [Google Scholar]
- 31.Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012;16:144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Morse SA, Zhang R, Thakur V, Reisin E. Hypertension and the metabolic syndrome. Am J Med Sci. 2005;330:303–310. doi: 10.1097/00000441-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Grassi G, Dell’Oro R, Facchini A, Quarti Trevano FD, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22:2363–2369. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Grassi G. Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertens Res Off J Jap Soc Hypertens. 2006;29:839–847. doi: 10.1291/hypres.29.839. [DOI] [PubMed] [Google Scholar]
- 35.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 36.Fu LW, Longhurst JC. Reflex pressor response to arterial phenylbiguanide: role of abdominal sympathetic visceral afferents. Am J Physiol. 1998;275:H2025–H2035. doi: 10.1152/ajpheart.1998.275.6.H2025. [DOI] [PubMed] [Google Scholar]
- 37.Bernal-Mizrachi C, Xiaozhong L, Yin L, Knutsen RH, Howard MJ, Arends JJ, Desantis P, Coleman T, Semenkovich CF. An afferent vagal nerve pathway links hepatic PPARalpha activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metab. 2007;5:91–102. doi: 10.1016/j.cmet.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor PM. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr. 2014;99:223S–230S. doi: 10.3945/ajcn.113.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310. doi: 10.1146/annurev.nutr.25.050304.092545. [DOI] [PubMed] [Google Scholar]
- 40.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chantranupong L, Wolfson RL, Sabatini DM. Nutrient-sensing mechanisms across evolution. Cell. 2015;161:67–83. doi: 10.1016/j.cell.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korsheninnikova E, van der Zon GC, Voshol PJ, Janssen GM, Havekes LM, Grefhorst A, Kuipers F, Reijngoud DJ, Romijn JA, Ouwens DM, Maassen JA. Sustained activation of the mammalian target of rapamycin nutrient sensing pathway is associated with hepatic insulin resistance, but not with steatosis, in mice. Diabetologia. 2006;49:3049–3057. doi: 10.1007/s00125-006-0439-5. [DOI] [PubMed] [Google Scholar]
- 44.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 45.Goberdhan DC, Wilson C, Harris AL. Amino acid sensing by mTORC1: intracellular transporters mark the spot. Cell Metab. 2016;23:580–9. [DOI] [PMC free article] [PubMed]
- 46.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr Opin Lipidol. 2012;23:226–234. doi: 10.1097/MOL.0b013e328352dd03. [DOI] [PubMed] [Google Scholar]
- 50.Ranganathan G, Pokrovskaya I, Ranganathan S, Kern PA. Role of A kinase anchor proteins in the tissue-specific regulation of lipoprotein lipase. Mol Endocrinol. 2005;19:2527–2534. doi: 10.1210/me.2005-0144. [DOI] [PubMed] [Google Scholar]
- 51.Brunzell JD. Clinical practice. Hypertriglyceridemia. New Engl J Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- 52.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]