Abstract
Endothelial dysfunction and tubulointerstitial fibrosis are characteristics of diabetic kidneys. Recent evidence has suggested that the diabetic kidney is associated with dipeptidyl peptidase (DPP)-4 overexpression in endothelial cells. Several insults can induce endothelial cells to alter their phenotype into a mesenchymal-like phenotype via endothelial–mesenchymal transition (EndMT), which plays pivotal roles in tissue fibrosis. We have recently revealed the fibrogenic role of DPP-4 through the induction of EndMT in diabetic kidneys. This review mainly focuses on the biological and pathological significance of DPP-4 overexpression in endothelial cells through the mechanisms of endothelial homeostasis defects, EndMT, and kidney fibrosis.
Keywords: Fibrosis, microRNA, Peptidase, Integrin, Extracellular matrix
Introduction
Approximately 45 % of all deaths in the developed world are caused by chronic fibrogenic disorders [1]. Kidney fibrosis, particularly tubulointerstitial fibrosis, is commonly the final outcome of progressive kidney diseases. Diabetes is also associated with progressive fibrosis of the kidney, as characterized by matrix deposition and glomerulosclerosis. Notably, fibrosis is initially a tissue-repairing process, and progressive kidney fibrosis may result from the disruption of the normal wound-healing process [1, 2]. It is difficult to determine the factor primarily responsible for this result, e.g., the cellular induction of fibrosis or the production of excess extracellular matrix. Many cell types are involved in the process, including resident cell types, such as resident fibroblasts, tubular epithelial cells, pericytes, endothelial cells, vascular smooth muscle cells, mesangial cells, and podocytes, but many nonresident bone marrow-derived cells/fibrocytes and inflammatory cells are also involved [3, 4]. During the progression of kidney fibrosis, each cell type, resident or nonresident, has a distinct role in the fibrogenic process, thus demonstrating cooperation among the different cell types. Additionally, the change from an epithelial or endothelial cell phenotype to a mesenchymal phenotype through epithelial–mesenchymal transition (EMT) or endothelial–mesenchymal transition (EndMT) may represent important programs generating matrix-producing mesenchymal-like cells [3, 4]. Diabetes is associated with endothelial damage by multiple insults, including those from transforming growth factor (TGF)-βs. Therefore, among the various processes of the mesenchymal programs mentioned above, we and others have focused on the EndMT program in the fibrosis of diabetic kidneys that is induced by TGF-βs.
Recently, we have found that dipeptidyl peptidase (DPP)-4, a molecule functioning in the maintenance of blood glucose levels via the modulation of incretin hormones, may play a vital role in endothelial cell homeostasis as well as extracellular matrix production. This review will focus on the biology of DPP-4 in endothelial cells and how this enzyme is a relevant therapeutic target for diabetic nephropathy and kidney fibrosis.
EndMT and TGF-β
The profibrotic cytokine TGF-β is produced by resident kidney cells and infiltrating inflammatory cells and is also filtered from the plasma during proteinuria [5]. The three isoforms of TGF-β (TGF-β1, TGF-β2, and TGF-β3) are ubiquitously expressed and play biologically significant roles in most cell types in mammals through the intracellular signaling cascade involving the Smad family of proteins. TGF-β type I (TβRI) and type II (TβRII) receptors are signaling receptors that form heteromeric cell surface complexes with TGF-βs as one of the earliest events in the cellular response [18]. EndMT is activated mainly by the TGF-β2 isoform [6–8]. The primary action of TGF-β1 on endothelial cell (EC)s is likely to induce cell proliferation [9]. This effect of TGF-β1 occurs through the TGF-β type III receptor, known as endoglin, on ECs [10]. Endoglin is an auxiliary receptor by which TGF-β signaling responses through TGF-β/ALK1 signaling are stimulated, whereas TGF-β/ALK5 signaling is inhibited [10]. In ECs, TGF-β1 binds to endoglin and induces signaling via ALK1 rather than ALK5. This TGF-β1/ALK1 interaction results in the phosphorylation of Smad1/5/8; subsequently, TGF-β1 signaling induces endothelial cell proliferation [9]. In ECs, TGF-β2 promotes signaling through ALK5 and ALK2, resulting in the phosphorylation of both Smad1/5/8 and Smad2/3, and also induces EndMT. EndMT has shown to be induced by TGF-β2 via Smad-independent pathways, including MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal regulated kinase) kinase], PI3K (phosphoinositide 3-kinase) and p38 MAPK.
DPP-4 and DPP-4 inhibitors
DPP-4 is a member of the serine peptidase/prolyl oligopeptidase gene family and was originally characterized as a T-cell differentiation antigen (CD26) and as a cell surface aminopeptidase. The DPP-4 gene encodes a type II transmembrane protein of 766 amino acids. DPP-4 is anchored to the lipid bilayer of the cell membrane by a single hydrophobic segment located at the N-terminus, and it has a short cytoplasmic tail of six amino acids [11]. The extracellular portion of DPP-4 contains glycosylated, cysteine-rich catalytic domains (Fig. 1) [12]. The C-terminal loop of DPP-4 is essential for its catalytic efficacy, and its dimerization was confirmed in a mutation study [13]. Additionally, catalytically active DPP-4 is detached (or cleaved) from the plasma membrane, resulting in the production of a soluble circulating form of DPP-4, sDPP-4 (727 aa).
Fig. 1.
Two forms of DPP-4: membrane-anchored and soluble. Catalytically active DPP-4 is cleaved from the plasma membrane, resulting in the production of the soluble circulating form, sDPP-4. sDPP-4, lacking both an intracellular tail and transmembrane regions, accounts for a substantial proportion of DPP-4 activity. In addition to peptidase activity, DPP-4 also acts as a binding protein with fibronectin and adenosine deaminase (ADA)
DPP-4 has the following diverse biological functions: protease activity; interaction with adenosine deaminase (ADA) and the extracellular matrix; co-receptor activity mediating viral entry; and regulation of intracellular signals associated with cell migration and/or proliferation [14–18]. DPP-4 is ubiquitously expressed and is found in diverse cell types, including endothelial cells in several organs. Interestingly, the enzymatic activity of DPP-4 is the highest in the kidney per organ weight. More than 30 substrates have been reported for DPP-4, including glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP) [12], brain natriuretic peptide 1–32 [19, 20], neuropeptide Y [21], high mobility group protein 1 (HMGΒ1) [22], and others [21, 23, 24]. Recently, two major growth factors of hematopoietic cells, erythropoietin and granulocyte-colony stimulating factor, have also been identified as substrates for DPP-4 [25]. The complexities of the biological activities of DPP-4 are indeed enhanced by this array of bioactive substances for DPP-4, thus highlighting the elegant role of DPP-4 in the biochemical tuning of multiple tissues.
DPP-4 inhibitors are well-recognized drugs for type 2 diabetes. In addition to their glucose-lowering action, DPP-4 inhibitors have been found in pre-clinical studies to exhibit cardiovascular protection against hypertension [26], abdominal aortic aneurysm [27], cardiomyopathy [28], atherosclerosis [29], and peripheral vascular disease [30] via both GLP-1-dependent and -independent pathways through their diverse, widely distributed, pleiotropic actions [31]. Furthermore, DPP-4 inhibitors have been shown to prevent fibrosis in diverse organs, including the heart [32, 33], liver [34], and kidney [35]. DPP-4 is expressed on the cell surface in many cell types, such as endothelial cells, kidney epithelial cells, and T cells. DPP-4 localizes in the cell membranes in diverse cell types, interacts with binding partners, and transmits intracellular signals [36]. The kidney is the organ in which DPP-4 is expressed at its highest level per organ weight [21]. Inflammation induces the expression of DPP-4 in human renal glomerular epithelial cells [37]. Additionally, diabetic conditions increase DPP-4 protein levels in rodent kidneys [35, 38]. Several candidates for the incretin hormone-independent effects of DPP-4 inhibitors in the kidney have been reported, and some reports have indicated that the known substrates of DPP-4, such as HMGΒ1, meprin β, neuropeptide Y (NPY), and peptide YY (PYY), play roles in its mechanisms [39]. Therefore, the increased activities of DPP-4 in the kidney or urine are potential hallmarks of human glomerular diseases [36, 40]. In rodent models, DPP-4 inhibitors mediate a reduction in urine albumin and improve histological alterations in the kidney in both type 1 and type 2 diabetes [35, 41–43]. In addition to the blockade of the Ang II receptor, linagliptin reduces urine albumin levels in diabetic endothelial nitric oxide synthase-knockout mice [44]. Similarly, linagliptin significantly reduces urinary albumin excretion in addition to the recommended standard treatment of patients with type 2 diabetes and renal damage [45]. The antifibrotic effects of DPP-4 inhibitors have also been reported in another nondiabetic kidney fibrosis model, unilateral ureteral obstruction (UUO). The DPP-4 inhibitor LC15-0444 significantly reduces urine albuminuria, urinary excretion of 8-isoprostane, and renal fibrosis in UUO kidneys [46]. In our recent study, we found that linagliptin restores the normal kidney structure in streptozotocin (STZ)-induced diabetic kidneys of CD-1 mice without altering blood sugar, blood pressure, body weight, or the weights of the kidney, liver, and heart [35].
We have shown that linagliptin suppresses kidney fibrosis in STZ-induced diabetic mice and is associated with the inhibition of EndMT as well as the suppression of DPP-4 activity, DPP-4 protein expression, and TGF-β-Smad signaling [35]. EndMT has been shown to be crucially important in forming the valves and septa of the heart during embryo development [47, 48]. EndMT may contribute to the accumulation of activated fibroblasts and myofibroblasts in kidney fibrosis [49]. In this regard, endothelium-specific heterozygous TGF-β receptor II knockout mice exhibit less tubulointerstitial fibrosis, enhanced preservation of renal microvasculature, improved renal blood flow, and less tissue hypoxia in UUO or folic acid nephropathy kidney fibrosis models [50]. EndMT has also been shown to be relevant in heart fibrosis and tumor stroma [51–56]. Serum from CKD patients, which contains a significant amount of a circulatory inhibitor of angiogenesis and nitric oxide, inhibits the proliferation of cardiac endothelial cells and induces apoptosis and the EndMT [57]. In this regard, the DPP-4 inhibitor linagliptin induces an antifibrotic effect through a mechanism that specifically targets endothelial cells [35, 58, 59]. We found that DPP-4 plays vital roles in TGF-β-stimulated signal transduction mechanisms by showing that the formation of the TGF-β2-induced TGF-βRI/II heterodimer is suppressed in DPP-4 siRNA-transfected endothelial cells [59]. These results indicate that DPP-4 overexpression in endothelial cells plays a pathogenic role by enhancing TGF-β signaling and EndMT.
The interaction of DPP-4 and integrin β1 in endothelial cells plays vital roles in the fibrogenic program
Integrins play vital roles in cell–cell or cell–matrix interactions through αβ heterodimers. There are 18 α-subunits and 8 β-subunits of integrins, and each exhibits different ligand binding and signaling properties [60]. Integrin subunits contain an extracellular domain, which determines their ligand-binding properties, a transmembrane domain, and a short cytoplasmic tail that binds to multiple cytosolic and transmembrane proteins by forming a focal adhesion complex (with the exception of β4) [61]. Integrins interact with extracellular matrix (ECM) glycoproteins (collagens, fibronectins, and laminins), cellular receptors (vascular cell adhesion molecule-1; VCAM-1) and members of the intracellular cell adhesion molecule (ICAM) family [62, 63]. Furthermore, integrins play vital roles in actin cytoskeleton remodeling as well as in tuning cell signals that regulate biological and cellular functions, such as cell adhesion, migration, proliferation, differentiation, and apoptosis [64]. Integrins have no catalytic site and no kinase activity, but they act as a bridge between the ECM and the actin cytoskeleton. This integrin-mediated interaction between the ECM and the actin cytoskeleton allows integrins to regulate cytoskeletal organization, cell motility, and many intracellular-signaling pathways, such as cell survival, cell proliferation, cell shape, and angiogenesis [65, 66]. Therefore, integrins play a vital role in maintaining the homeostasis of organ functions under normal conditions and in pathogenic conditions. Integrins display significant roles in several fibroproliferative diseases, including diabetic nephropathy [67–70].
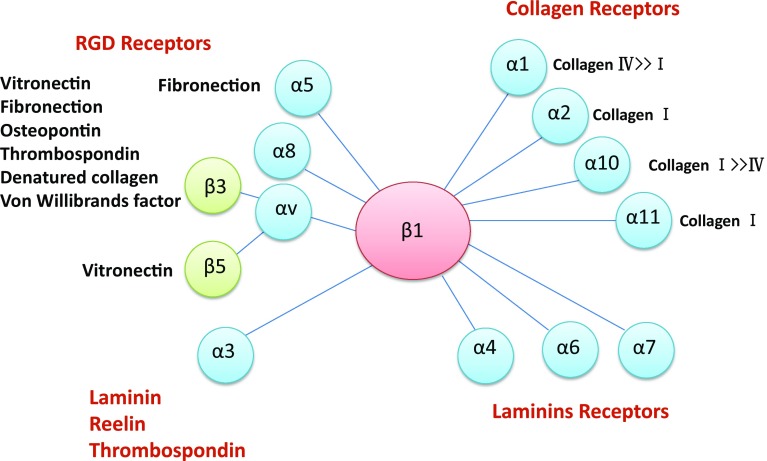
Integrin β1 can form a heterodimer with at least 11 α-subunits. Thus, integrin β1 acts as a receptor for specific ECM components [71–74] (Fig. 2). Integrin β1 is ubiquitously expressed in embryonic development through adulthood. Therefore, it is not surprising that integrin β1 knockout mice are embryonic lethal due to a failure in preimplantation development. Podocyte-specific integrin β1 knockout mice are delivered normal. After birth, however, they develop a remarkable amount of proteinuria with podocyte defects and die within the first week of life [75]. In fibroblasts, the expression of integrin β1 is required for fibrogenesis, and blockade of integrin β1 signaling can diminish the progression of cutaneous fibrosis [76]. In the UUO kidney, both the mRNA and protein expression of integrin β1 are significantly induced and are associated with the levels of tubular TGF-β1 [69]. The activation of integrin β1 is a pivotal event for the induction of TGF-β1, and the inhibition of integrin β1 results in the reduction in TGF-β1 levels, thus suggesting significant crosstalk between integrin β1 and TGF-β signaling in the pathogenesis of tubulointerstitial fibrosis [69].
Fig. 2.
Integrin β1 receptors and their ligands. In the integrin family, integrin β1 is the most critical member because integrin β1 can form heterodimers with at least 11 types of α-subunits, making them receptors for many types of stimuli and ligands
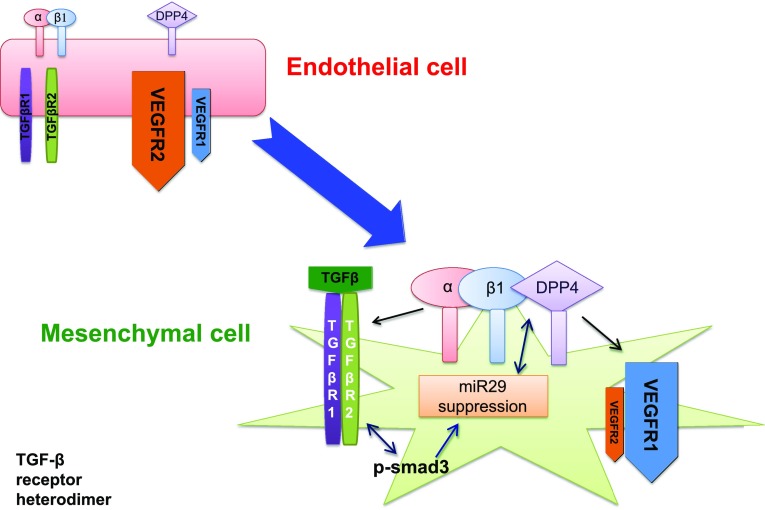
The loss of membrane-bound DPP-4 has shown to be associated with a reduction in the phosphorylation of integrin β1 at residue S785, which has a key role in the cellular adhesion of integrin β1 to the ECM [77]. Regarding this finding, we have recently reported a novel profibrotic molecular mechanism mediated by the interaction of DPP-4 and integrin β1 [59] (Fig. 3). TGF-β2-induced EndMT was associated with the induction of DPP-4 and integrin β1. In cultured endothelial cells; the incubation of activating antibody for integrin β1 induced DPP-4 protein expression associated with EndMT. DPP-4 and integrin β1 exhibited a physical interaction as determined by a close proximity assay and immunoprecipitation analysis. DPP-4 suppression by siRNA resulted in the suppression of integrin β1 and vice versa. Either integrin β1 or DPP-4 knockdown resulted in the inhibition of TGF-β2-induced TGF-β receptor heterodimer formation, Smad3 phosphorylation, and EndMT. Furthermore, the interaction between DPP-4 and integrin β1 induces the expression of vascular endothelial growth factor receptor (VEGFR) 1, a type of “decoy” receptor for VEGF-induced angiogenesis events, and it results in the concomitant suppression of the VEGFR2 angiogenic receptor [59]. Such alterations in VEGF receptors are relevant because VEGF is a potent stimulator of angiogenesis in endothelial cells, mainly through VEGFR2. The actions of VEGF on endothelial cells are regulated by several VEGF receptors, each of which plays distinct roles in EndMT. VEGFR1 favors EndMT, whereas VEGFR2 counteracts EndMT [7]. Indeed, in STZ-induced diabetic mice, DPP-4, integrin β1, and VEGF-R1 are all induced in endothelial cells, while VEGFR2 is suppressed. These data clearly demonstrate that the interaction of DPP-4 and integrin β1 may be a therapeutic target for maintaining the homeostasis of endothelial cells and treating kidney fibrosis in diabetes [59].
Fig. 3.
DPP-4 and integrin β1 interaction facilitates endothelial cell transition into the mesenchymal phenotype. Interaction between DPP-4 and integrin β1 induces TGF-β receptor heterodimer formation. TGF-β-receptor-activated Smad3 induces key mesenchymal programs in endothelial cells and also suppresses miR-29s. Suppression of mi-R29s further stimulates the expression of both integrin β1 and DPP-4 levels. Induction of integrin β1 also induces VEGFR1, the decoy receptor for VEGF-mediated survival signaling in endothelial cells and angiogenesis. In contrast, VEGFR2, the angiogenic receptor for VEGF, is suppressed. These events induce the conversion of endothelial cells into mesenchymal cells
MicroRNAs and DPP-4 in the kidney
MicroRNAs (miRs) have been intensively analyzed for their biological significance for the past 20 years. The expression profiles of miRNAs are altered by several physiological/environmental stimuli, such as sustained hyperglycemia, inflammatory cytokines, proteinuria, ageing, high blood pressure, and hypoxia, which result in alterations in the kidney miRNA expression profiles. Such alterations in miRNA levels may induce the transition program in the normal kidney, thus leading to fibrosis. The synthesis and levels of miRs are tightly regulated. The known antifibrotic miRs, miRNA-29s, are involved in the protection of diabetic kidneys from the fibrotic process [35, 78, 79]. By screening miRNA targets with TargetScan (http://www.targetscan.org/vert_60/), we identified a conserved miRNA-29 binding site in the 3′UTR of DPP-4 [35]. By cloning and utilizing the 3′UTR of the human DPP-4 mRNA in a reporter vector, we experimentally showed that the miR-29-binding site in the DPP-4 3′UTR functionally suppresses DPP-4 gene expression. In the kidneys of diabetic mice, miR-29 levels are suppressed compared with those of normoglycemic mice [35] (Fig. 4). Linagliptin ameliorates the kidney histology and functions associated with the induction of miR-29 expression in diabetic kidneys [35]. Additionally, linagliptin treatment restored the levels of miR-29s that were suppressed by TGF-β2 in primary cultured endothelial cells (Fig. 4). Importantly, miR-29 also targets integrin β1 [80, 81]; therefore, the levels of miR-29s can directly affect the levels of both DPP-4 and integrin β1. Furthermore, very recently, we have shown [82] that miR-29s and miR-let-7s, another miR, play anti-EndMT roles [83] consisting of anti-EndMT crosstalk regulation against the mesenchymal activation program in vivo and in vitro. This type of bidirectional regulation between two miRs could play an essential role in maintaining the anti-EndMT program [82]. Therefore, DPP-4-inhibition-associated induction of miR-29s can be relevant to the suppression of EndMT programs via the suppression of miR-29 target genes such as DPP-4 and integrin β1, as well as the induction of anti-EndMT miRs crosstalk.
Fig. 4.
Biological effects of miR-29 on the ECM and DPP-4. miR-29 has been shown to be a negative regulator of several ECM genes, including integrin β1. TGF-β2 suppresses miR-29 levels. Therefore, extracellular matrix genes, which have a miR-29-binding site in their 3′UTR, are induced by TGF-β2. We have identified the functional miR-29-binding site in the 3′UTR of the DPP-4 gene
Perspective
In clinical settings, it is not clear how the DPP-4 inhibitors differ. The glucose-lowering effects of the DPP-4 inhibitors are likely to be similar. However, each DPP-4 inhibitor has a distinct chemical structure. The diversity of DPP-4 inhibitors results in unique pharmacodynamic and pharmacokinetic profiles, and the structural diversity of DPP-4 inhibitors may influence the pleiotropic effects of the DPP-4 inhibitors. Utilizing two distinct DPP-4 inhibitors, sitagliptin and linagliptin, we recently found that linagliptin inhibits TGF-β-induced Smad3 phosphorylation, miR-29 suppression, the induction of VEGFR1, EndMT, and the interaction between DPP-4 and integrin β1 [84]. However, sitagliptin does not inhibit any of these processes [84]. Interestingly, both linagliptin and sitagliptin inhibit DPP-4 activity in a medium containing recombinant DPP-4 in a cell-free system. TGF-β2-induced DPP-4 activity in cultured endothelial cells is inhibited by linagliptin but not by sitagliptin, thus suggesting that only linagliptin can inhibit the molecular crosstalk involved in EndMT. To explain the molecular mechanisms of such differences between these two DPP-4 inhibitors, we have observed that linagliptin inhibits DPP-4 homodimerization, which supports the enzymatic activity of DPP-4, whereas sitagliptin does not inhibit DPP-4 homodimerization. Several potential reasons for the differences between these DPP-4 inhibitors are described below. (i) DPP-4 inhibitors can be divided on the basis of their structure into those that mimic the dipeptide structure of DPP-4 substrates and those that are nonpeptidomimetic. Sitagliptin belongs to the peptidomimetic family, which exhibits a triazolopiperazine-based structure, and linagliptin belongs to the nonpeptidomimetic family, which includes imidazole-based inhibitors [85, 86]. (ii) Linagliptin is likely lipophilic [87]. Sitagliptin exhibits pH-dependent aqueous solubility. Sitagliptin is soluble in water and N,N-dimethyl formamide, and it is slightly soluble in methanol [88]. (iii) Recently, DPP-4 inhibitors have also been divided into three classes according to their binding subsites [89]. Linagliptin belongs to the second class and interacts with the S1 and S2 subsites, but it also interacts with the additional S′1 subsite where Tyr547 is located. Among the DPP-4 inhibitors, only linagliptin binds to the S′2 subsite. Sitagliptin belongs to the third class and binds to the S1, S2, and S2extensive subsites [89]. (iv) The dimerization of DPP-4 is required for its activity, and Tyr547 (linagliptin binding site), Glu205, and Glu206 from the propeller loop are critical for its dimerization [90–92]. It is not entirely clear how the differences in these DPP-4 inhibitors might affect DPP-4-homodimer formation at the cell membrane. DPP-4 inhibitors might directly interfere with DPP-4 homodimer formation. Another possibility is that DPP-4 inhibition may affect the intracellular signaling cascade that is required for DPP-4 homodimer formation. Further study is required to clearly determine the molecular mechanisms of how each DPP-4 inhibitor affects the DPP-4 activity at the cell membrane. Additionally, clinical studies must further evaluate whether such differences in DPP-4 inhibitors have different clinical effects.
Conclusions
This review describes the antifibrotic effects of DPP-4 inhibitors with a focus on the role of DPP-4 overexpression in endothelial cells and EndMT. The potential antifibrotic effects of DPP-4 inhibitors are likely to be beneficial for human health in the context of diabetic patients. However, fibrosis is a tissue-repair process. Without adequate amelioration of tissue-damaging insults, such as hyperglycemia, hypertension, and inflammation, “antifibrosis” treatment alone would result in deficiencies in the tissue-repair process and perhaps induce further tissue injury. Additionally, there are other pleotropic effects of DPP-4 inhibitors, and some are not always favorable [93–95]. Therefore, the biology of DPP-4 must be further understood, and it remains to be determined how the pleiotropic effects of DPP-4 inhibitors might contribute to human health.
Acknowledgments
Parts of this review were presented in the Lilly Award Lecture at the Japan Diabetes Society 2016, Kyoto, Japan. KK would like to express his sincere gratitude to Professor Daisuke Koya for his guidance and support. This work was partially supported by grants from the Japan Society for the Promotion of Science to KK (23790381) and research grants from the Japan Research Foundation for Clinical Pharmacology to KK (2011). This work was partially supported by a Grant for Promoted Research awarded to KK (S2013-13, S2014-4, S2015-3) from Kanazawa Medical University.
Conflicts of interest
KK received lecture fees from Boehringer Ingelheim and Eli Lilly. Both Boehringer Ingelheim and Eli Lilly donated funds to Kanazawa Medical University and were not directly associated with this project. Boehringer Ingelheim, Mitsubishi Tanabe Pharma, and Ono Pharmaceutical contributed funds to establish the Division of Anticipatory Molecular Food Science and Technology. KK is in a consulting contract with Boehringer Ingelheim.
Ethics policy
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Pinzani M. Welcome to fibrogenesis and tissue repair. Fibrogenesis Tissue Repair. 2008;1:1. doi: 10.1186/1755-1536-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 4.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 5.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 6.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 7.Medici D, et al. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medici D, Potenta S, Kalluri R. Transforming growth factor-beta2 promotes Snail-mediated endothelial–mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J. 2011;437:515–520. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 11.De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–375. doi: 10.1016/S0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 12.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien CH, et al. One site mutation disrupts dimer formation in human DPP-IV proteins. J Biol Chem. 2004;279:52338–52345. doi: 10.1074/jbc.M406185200. [DOI] [PubMed] [Google Scholar]
- 14.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 15.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 17.Lu G, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahne T, et al. Dipeptidyl peptidase IV: a cell surface peptidase involved in regulating T cell growth (review) Int J Mol Med. 1999;4:3–15. doi: 10.3892/ijmm.4.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 20.Brandt I, et al. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 21.Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. [DOI] [PubMed]
- 22.Marchetti C, et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia. 2012;55:236–244. doi: 10.1007/s00125-011-2213-6. [DOI] [PubMed] [Google Scholar]
- 23.Kirby M, Yu DM, O’Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci (Lond) 2010;118:31–41. doi: 10.1042/CS20090047. [DOI] [PubMed] [Google Scholar]
- 24.Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54:249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 25.Broxmeyer HE, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–1796. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira L, et al. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat) Mediat Inflamm. 2010;2010:592760. doi: 10.1155/2010/592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu HY, et al. Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS One. 2015;10:e0121077. doi: 10.1371/journal.pone.0121077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 29.Shah Z, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CY, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012;167:1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cernea S, Raz I. Therapy in the early stage: incretins. Diabetes Care. 2011;34(Suppl 2):S264–S271. doi: 10.2337/dc11-s223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bostick B, et al. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63:1000–1011. doi: 10.1016/j.metabol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirakawa H, et al. A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS One. 2015;10:e0119360. doi: 10.1371/journal.pone.0119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaji K, et al. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol. 2013 doi: 10.1007/s00535-013-0783-4. [DOI] [PubMed] [Google Scholar]
- 35.Gao C, Huang W, Kanasaki K, Xu Y. The role of ubiquitination and sumoylation in diabetic nephropathy. Biomed Res Int. 2014;2014:160692. doi: 10.1155/2014/160692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun AL, et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diabetes Vasc Dis Res. 2012;9:301–308. doi: 10.1177/1479164111434318. [DOI] [PubMed] [Google Scholar]
- 37.Stefanovic V, et al. Interferon-gamma induces dipeptidylpeptidase IV expression in human glomerular epithelial cells. Immunology. 1993;80:465–470. [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, et al. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci. 2007;81:272–279. doi: 10.1016/j.lfs.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Panchapakesan U, Mather A, Pollock C. Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin Sci (Lond) 2013;124:17–26. doi: 10.1042/CS20120167. [DOI] [PubMed] [Google Scholar]
- 40.Mitic B, Lazarevic G, Vlahovic P, Rajic M, Stefanovic V. Diagnostic value of the aminopeptidase N,N-acetyl-beta-d-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren Fail. 2008;30:896–903. doi: 10.1080/08860220802359048. [DOI] [PubMed] [Google Scholar]
- 41.Mega C, et al. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat) Exp Diabetes Res. 2011;2011:162092. doi: 10.1155/2011/162092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu WJ, et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012;340:248–255. doi: 10.1124/jpet.111.186866. [DOI] [PubMed] [Google Scholar]
- 43.GangadharanKomala M, Gross S, Zaky A, Pollock C, Panchapakesan U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology (Carlton) 2016;21:423–431. doi: 10.1111/nep.12618. [DOI] [PubMed] [Google Scholar]
- 44.Alter ML, et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res. 2012;36:119–130. doi: 10.1159/000341487. [DOI] [PubMed] [Google Scholar]
- 45.Groop PH, et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min HS, et al. Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction. Lab Invest. 2014;94:598–607. doi: 10.1038/labinvest.2014.50. [DOI] [PubMed] [Google Scholar]
- 47.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.RES.77.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial–mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xavier S, et al. Curtailing endothelial TGF-beta signaling is sufficient to reduce endothelial–mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015;26:817–829. doi: 10.1681/ASN.2013101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 52.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 53.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, et al. Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circ Res. 2015;116:857–866. doi: 10.1161/CIRCRESAHA.116.305629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X, et al. Epigenetic balance of aberrant Rasal1 promoter methylation and hydroxymethylation regulates cardiac fibrosis. Cardiovasc Res. 2015;105:279–291. doi: 10.1093/cvr/cvv015. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, et al. Snail is a direct target of hypoxia-inducible factor 1alpha (HIF1alpha) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J Biol Chem. 2015;290:16653–16664. doi: 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charytan DM, et al. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol. 2014;176:99–109. doi: 10.1016/j.ijcard.2014.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeisberg M, Zeisberg EM. Evidence for antifibrotic incretin-independent effects of the DPP-4 inhibitor linagliptin. Kidney Int. 2015;88:429–431. doi: 10.1038/ki.2015.175. [DOI] [PubMed] [Google Scholar]
- 59.Shi S, et al. Interactions of DPP-4 and integrin beta1 influences endothelial-to-mesenchymal transition. Kidney Int. 2015;88:479–489. doi: 10.1038/ki.2015.103. [DOI] [PubMed] [Google Scholar]
- 60.Pozzi A, Zent R. Extracellular matrix receptors in branched organs. Curr Opin Cell Biol. 2011;23:547–553. doi: 10.1016/j.ceb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pozzi A, Zent R. Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Exp Nephrol. 2003;94:e77–84. doi:10.1159/000072025. [DOI] [PubMed]
- 62.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 63.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 64.Park EJ, Yuki Y, Kiyono H, Shimaoka M. Structural basis of blocking integrin activation and deactivation for anti-inflammation. J Biomed Sci. 2015;22:51. doi: 10.1186/s12929-015-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zent R, et al. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi: 10.1038/sj.ki.5000359. [DOI] [PubMed] [Google Scholar]
- 68.Chen X, et al. Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol. 2007;27:3313–3326. doi: 10.1128/MCB.01476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeh YC, et al. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamzeh MT, Sridhara R, Alexander LD. Cyclic stretch-induced TGF-beta1 and fibronectin expression is mediated by beta1-integrin through c-Src- and STAT3-dependent pathways in renal epithelial cells. Am J Physiol Renal Physiol. 2015;308:F425–F436. doi: 10.1152/ajprenal.00589.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang XH, Huang SM, Tan SQ, Ma YL. Effect of rosiglitazone on integrin beta1 expression and apoptosis of proximal tubular cell exposed to high glucose. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:291–294. [PubMed] [Google Scholar]
- 72.Glynne PA, Picot J, Evans TJ. Coexpressed nitric oxide synthase and apical beta(1) integrins influence tubule cell adhesion after cytokine-induced injury. J Am Soc Nephrol. 2001;12:2370–2383. doi: 10.1681/ASN.V12112370. [DOI] [PubMed] [Google Scholar]
- 73.Elias BC, et al. The integrin beta1 subunit regulates paracellular permeability of kidney proximal tubule cells. J Biol Chem. 2014;289:8532–8544. doi: 10.1074/jbc.M113.526509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lal H, et al. Integrins and proximal signaling mechanisms in cardiovascular disease. Front Biosci (Landmark Ed) 2009;14:2307–2334. doi: 10.2741/3381. [DOI] [PubMed] [Google Scholar]
- 75.Kanasaki K, et al. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A. Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 2009;60:2817–2821. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 77.Sato T, et al. CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta1, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299. Cancer Res. 2005;65:6950–6956. doi: 10.1158/0008-5472.CAN-05-0647. [DOI] [PubMed] [Google Scholar]
- 78.Srivastava SP, Koya D, Kanasaki K. MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT. Biomed Res Int. 2013;2013:125469. doi: 10.1155/2013/125469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nitta K, et al. Oral administration of N-acetyl-seryl-aspartyl-lysyl-proline ameliorates kidney disease in both type 1 and type 2 diabetic mice via a therapeutic regimen. Biomed Res Int. 2016;2016:9172157. doi:10.1155/2016/9172157. [DOI] [PMC free article] [PubMed]
- 80.Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, et al. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2) PLoS One. 2013;8:e70192. doi: 10.1371/journal.pone.0070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Srivastava SP, et al. Effect of antifibrotic microRNAs crosstalk on the action of N-acetyl-seryl-aspartyl-lysyl-proline in diabetes-related kidney fibrosis. Sci Rep. 2016;6:29884. doi:10.1038/srep29884. [DOI] [PMC free article] [PubMed]
- 83.Chen PY, et al. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Reports. 2012;2:1684–96. doi:10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed]
- 84.Shi S, Kanasaki K, Koya D. Linagliptin but not sitagliptin inhibited transforming growth factor-beta2-induced endothelial DPP-4 activity and the endothelial–mesenchymal transition. Biochem Biophys Res Commun. 2016;471:184–90. doi:10.1016/j.bbrc.2016.01.154. [DOI] [PubMed]
- 85.Havale SH, Pal M. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg Med Chem. 2009;17:1783–1802. doi: 10.1016/j.bmc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Hu Y, Liu T. Recent advances in non-peptidomimetic dipeptidyl peptidase 4 inhibitors: medicinal chemistry and preclinical aspects. Curr Med Chem. 2012;19:3982–3999. doi: 10.2174/092986712802002491. [DOI] [PubMed] [Google Scholar]
- 87.Terawaki Y, et al. Efficacy of dipeptidyl peptidase-4 inhibitor linagliptin in patients with type 2 diabetes undergoing hemodialysis. Diabetol Metab Syndr. 2015;7:44. doi: 10.1186/s13098-015-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzpatrick S, Taylor S, Booth SW, Newton MJ. The development of a stable, coated pellet formulation of a water-sensitive drug, a case study: development of a stable core formulation. Pharm Dev Technol. 2006;11:521–528. doi: 10.1080/10837450600941079. [DOI] [PubMed] [Google Scholar]
- 89.Nabeno M, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434:191–196. doi: 10.1016/j.bbrc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Tang HK, et al. Role of a propeller loop in the quaternary structure and enzymatic activity of prolyl dipeptidases DPP-IV and DPP9. FEBS Lett. 2011;585:3409–3414. doi: 10.1016/j.febslet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999;266:798–810. doi: 10.1046/j.1432-1327.1999.00902.x. [DOI] [PubMed] [Google Scholar]
- 92.Bjelke JR, et al. Tyrosine 547 constitutes an essential part of the catalytic mechanism of dipeptidyl peptidase IV. J Biol Chem. 2004;279:34691–34697. doi: 10.1074/jbc.M405400200. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci Transl Med. 2016;8:334ra351. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
- 94.Kanasaki K, et al. Three ileus cases associated with the use of dipeptidyl peptidase-4 inhibitors in diabetic patients. J Diabetes Investig. 2013;4:673–675. doi: 10.1111/jdi.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Attaway A, Mersfelder TL, Vaishnav S, Baker JK. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors. A case report and review of literature. J Dermatol Case Rep. 2014;8:24–28. doi: 10.3315/jdcr.2014.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]