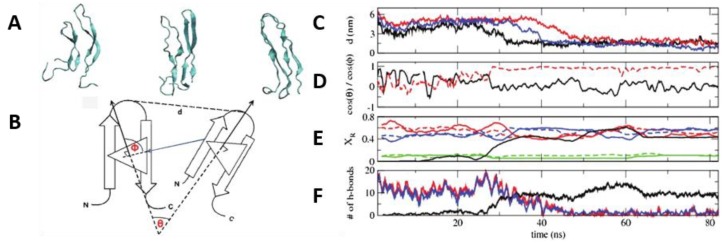
Figure 13.
Characterisation of the formation of dimers of hIAPP. (A) Representative configurations along the dimerisation pathway of two human amylin peptides in their β-hairpin conformation. The time evolution of the process of dimerisation was characterised, taking the distance between monomers using the turn-forming Asn22 (B), the angles between orientation vectors ((C,D) with schematic representation (B)) (first, molecules at a distance of 5 nm, with a θ angle between the two peptides of 90° (D, shown in black); second, molecules at a distance of 6 nm, with a θ angle between them of 180° (shown in red)) in each monomer, secondary structure (E), the number of main chain intra-strand hydrogen bonds in the two monomers, and the number of main chain inter-strand hydrogen bonds (F). Adapted from Reddy et al. [69]. Copyright (2010) with permission from Elsevier.