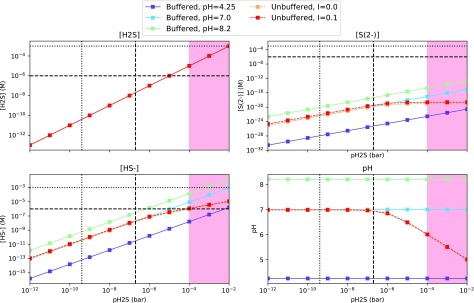
FIG. 1.
Concentrations of sulfur-bearing compounds and pH as a function of pH2S for a well-mixed aqueous reservoir. [H2S] is calculated from Henry's law; the concentrations of HS− and S2− are calculated from equilibrium chemistry for (1) solutions buffered to various pH values and (2) unbuffered solutions with varying ionic strengths. The vertical dotted line demarcates the expected pH2S for an abiotic Earth with a weakly reducing CO2-N2 atmosphere with modern levels of sulfur outgassing, from Hu et al. (2013). The vertical dashed line demarcates the expected pH2S for the same model but with outgassing levels of sulfur corresponding to the upper limit of the estimate for the emplacement of the terrestrial flood basalts. In the red shaded area, pH2S is so high it blocks UV light from the planet surface, meaning UV-dependent prebiotic pathways, e.g., those of Patel et al. (2015), cannot function (Ranjan and Sasselov, 2017). The red curve largely overplots the orange, demonstrating the minimal impact of ionic strength on the calculation for I ≤ 0.1. The horizontal dashed and dotted lines demarcate micromolar and millimolar concentrations, respectively. The cyanosulfidic chemistry of Patel et al. (2015) has been demonstrated at millimolar S-bearing photoreductant concentrations, and at least high micromolar levels of these compounds are thought to be required for high-yield prebiotic chemistry.