Table 1.
Selected optimization experiments in the enantioselective intermolecular ruthenium catalyzed cycloaddition of benzocyclobutenones.a
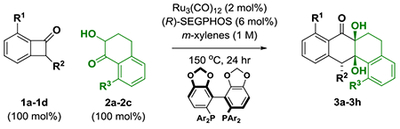
| entry | R1 | R2 | 1a-1d | R3 | 2a-2c | 3a-3h | yield (%), dr, rr | er |
|---|---|---|---|---|---|---|---|---|
| 1b | H | H | 1a | H | 2a | 3a | 87, >20:1, >20:1 | 53:47 |
| 2b | OMe | H | 1b | H | 2a | 3b | 83, >20:1, >20:1 | 81:19 |
| 3 | OMe | H | 1b | OMe | 2b | 3c | 77, >20:1, >20:1 | 58:42 |
| 4 | OMe | H | 1b | OBn | 2c | 3d | 79, >20:1, >20:1 | 76:24 |
| 5b | OMe | OTIPS | 1c | H | 2a | 3e | 75, >20:1, >20:1 | 87:13 |
| 6 | OMe | OTIPS | 1c | OMe | 2b | 3f | 78, >20:1, >20:1 | 89:11 |
| 7 | OMe | OTBS | 1d | OMe | 2b | 3g | 74, >20:1, >20:1 | 80:20 |
| 8 | OMe | OTIPS | 1c | OBn | 2c | 3h | 79, >20:1, >20:1 | 94:6 |
| ➡ 9c | OMe | OTIPS | 1c | OBn | 2c | 3h | 96, >20:1, >20:1 | 97:3 |
Yields are of material isolated by silica gel chromatography. Enantioselectivity values were determined by HPLC analysis on a chiral stationary phase. (R)-SEGPHOS (Ar = phenyl).
Reaction was conducted from the diol oxidation level, 1a-1c (200 mol%).
1c (130 mol%), (R)-DM-SEGPEIOS (Ar = 3,5-xylyl). See the Supporting Information for further experimental details.
