Figure 1.
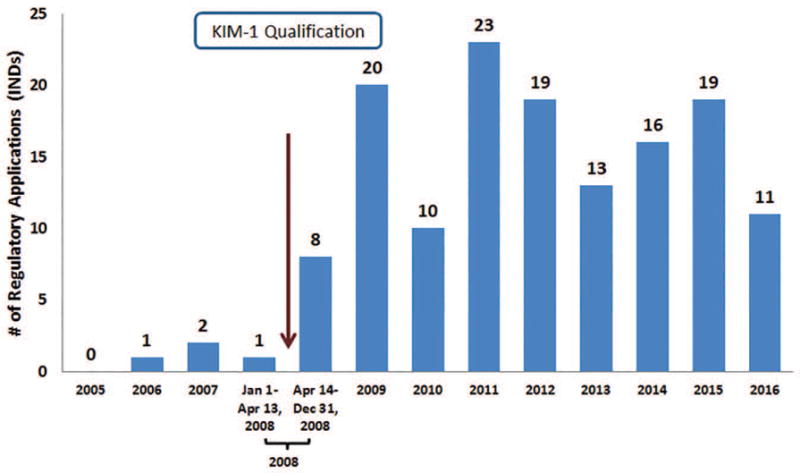
Number of regulatory applications (INDs) with FDA- generated documents in the DARRTS database that referenced KIM-1 as a drug development tool from 2005–2016.

Number of regulatory applications (INDs) with FDA- generated documents in the DARRTS database that referenced KIM-1 as a drug development tool from 2005–2016.