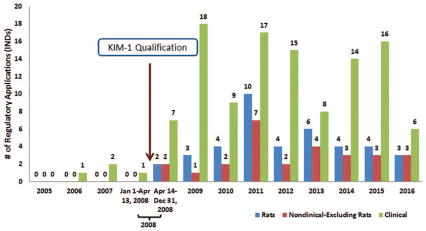
Figure 4.
Regulatory applications (INDs) with FDA- generated documents in the DARRTS database that referred to KIM-1 as a drug development tool, grouped by study type: rats, nonclinical (excluding rats), and clinical. Please note that a single regulatory document may contain multiple uses of KIM-1 (for example, use of KIM-1 in the nonclinical as well as clinical studies).