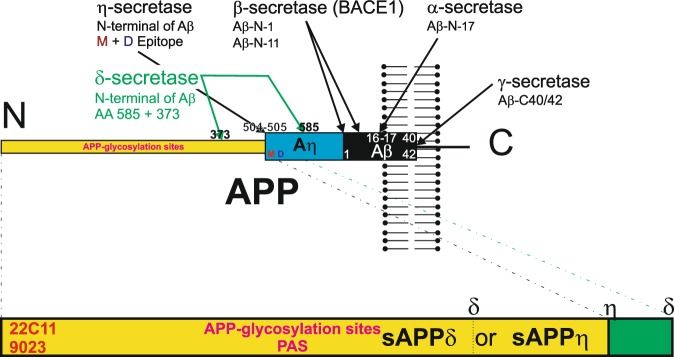
Figure 1.
Schematic representation of APP cleavage by α-, β-, γ-, δ- and η-secretase and generation of sAPPδ and sAPPη. The δ-secretase can thereby cut at the amino acid position 373 as well as at position 585. As such, δ-secretase cleavage can produce a longer N-terminal fragment that is detectable with antibodies against the D- and M-epitope sAPPδ585 and fragments that do not contain these epitopes, i.e. sAPPδ37317. sAPPδ373 and sAPPη can be detected with antibodies against the N-terminus of APP (22C11, 9023) but not with antibodies detecting APP C-terminal to the δ373 or η-secretase cleavage site. sAPPδ/η contain glycosylation sites possibly explaining the detection of its glycosylated form stained by the PAS-method. The antibodies used for APP-staining do not differentiate between sAPPδ373, sAPPη and shorter N-terminal fragments of APP.