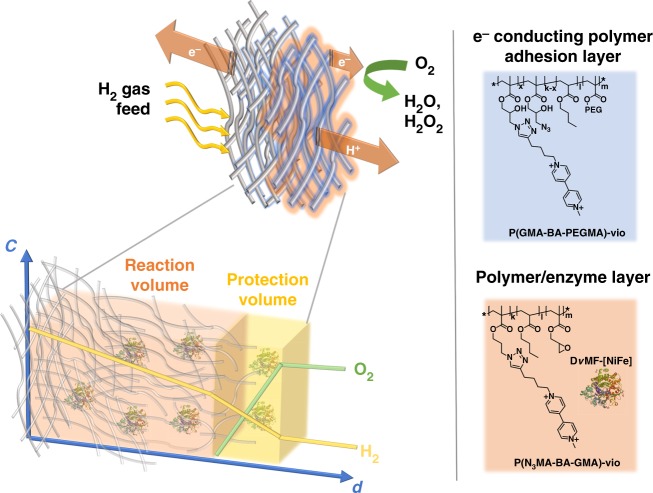
Fig. 1.
Schematic of the proposed gas-breathing polymer-based bioanode. The hydrogenase is electrically wired to the electrode surface via the viologen-modified redox polymers P(N3MA-BA-GMA)-vio (poly(3-azido-propyl methacrylate-co-butyl acrylate-co-glycidyl methacrylate)-viologen) and P(GMA-BA-PEGMA)-vio (poly(glycidyl methacrylate-co-butyl acrylate-co-poly(ethylene glycol) methacrylate)-viologen, blue). The latter acts additionally as adhesion layer for the P(N3MA-BA-GMA)-vio/hydrogenase reaction layer (pale red) due to its more hydrophobic character that enhances interactions with the hydrophobic surface of the carbon cloth gas diffusion electrode and prevents direct contact of the biocatalyst with the electrode surface, hence protecting the enzyme from high-potential deactivation (in combination with P(N3MA-BA-GMA)-vio) as well as unwanted contribution from DET. At the polymer/electrolyte interface incoming O2 is scavenged by the reduced polymer-bound viologen moieties (protection volume, yellow, left bottom). Expected normalized concentration profiles for H2 (yellow) and O2 (green) under turnover conditions are shown at the bottom (left) of the scheme illustrating protection from O2 (with c = concentrations and d = distance from electrode). O2 is reduced at the outer polymer/enzyme layer and hence the reaction layer remains unaffected. The porous structure of the carbon cloth-based electrode ensures a high polymer/biocatalyst loading and minimizes limitations due to slow electron transfer by keeping the electron transfer pathways short (see main text for further details). Note that the reaction as well as the protection layer contains the redox polymer and the biocatalyst. Not drawn to scale. DvMF-[NiFe] = [NiFe] hydrogenase from Desulfovibrio vulgaris Miyazaki F (4U9H)41. PEG poly(ethylene glycol)