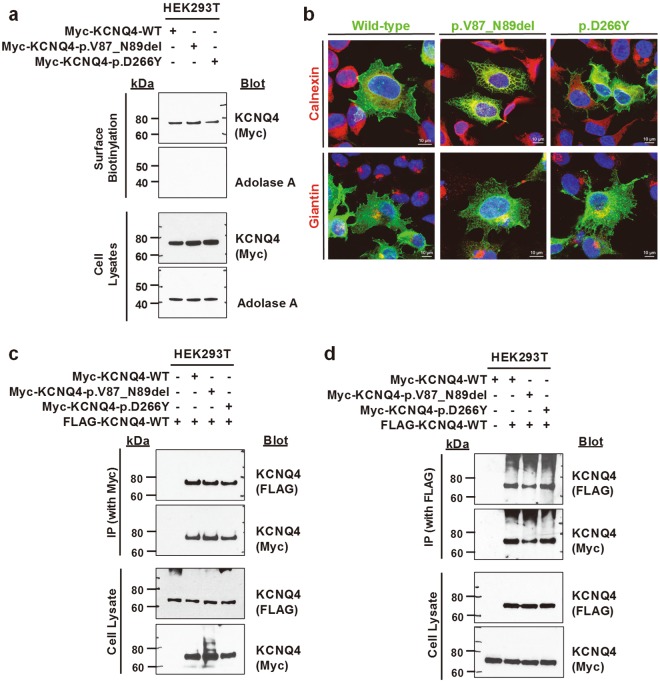
Figure 2.
Surface expression and subunit interactions of WT and mutant KCNQ4 proteins. HEK293T or HeLa cells were transfected with N-terminally Myc- or Flag-tagged WT and mutant KCNQ4 clones. (a) Cell surface biotinylation in HEK293T. Proteins on the plasma membrane were labelled with biotin, isolated with avidin beads, and assessed by western blotting. Surface expression of two mutant KCNQ4 proteins was similar to that of WT protein. (b) Immunofluorescence of WT and mutant KCNQ4 proteins in HeLa cells. Cells were immunostained with anti-Myc, anti-calnexin, and anti-giantin antibodies. Nuclei were stained with DAPI. Calnexin and giantin are markers for the endoplasmic reticulum and Golgi apparatus, respectively. Both mutant KCNQ4 proteins and WT protein were observed on the plasma membrane. (c–d) Subunit interactions between WT and mutant KCNQ4 proteins. Twenty-four hours post-transfection, whole-cell lysates were subjected to immunoprecipitation using anti-Myc (c) or anti-FLAG (d) antibodies and immunoblotted. Both KCNQ4 mutant proteins interacted with WT protein.