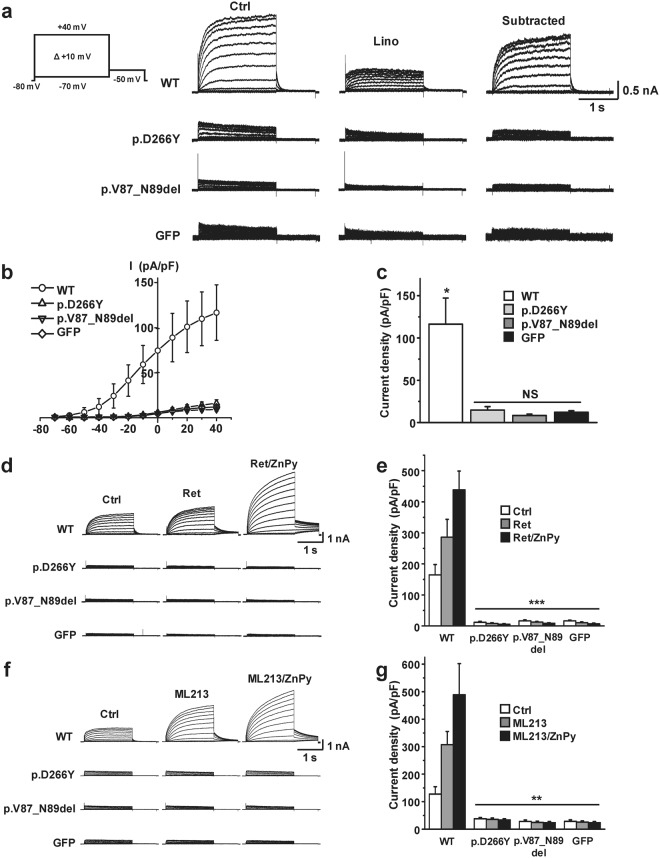
Figure 3.
Impaired potassium conductance of homomeric mutant KCNQ4 channels. (a) Whole-cell KCNQ4 K+ current traces recorded from the HEK293T cells transiently expressing WT, p.D266Y, p.V87_N89del, or GFP. Homomeric p.Asp266Tyr and p.Val87_Asn89del mutant channels produced barely detectable K+ currents. (b,c) Current-voltage (I-V) relationships of linopirdine (30 μM)-sensitive K+ currents (b), and current densities measured at +40 mV (c). The I-V curve of the WT protein exhibited a typical outwardly rectifying KCNQ4 channel current, and the current densities of p.D266Y and p.V87_N89del mutants were not significant (NS) compared with GFP. WT, n = 24; p.D266Y, n = 10; p.V87_N89del, n = 10; GFP, n = 22. *P < 0.05 versus mutants and GFP. (d–g) Homomeric mutant channels were not activated by known KCNQ openers. Single or combination treatment with retigabine (Ret, 10 μM), ML213 (3 μM), and zinc pyrithione (ZnPy, 10 μM) did not activate mutant channels (d,f), and the current densities after treating KCNQ openers were not significantly different from that of GFP (e,g). Mean ± SEM, n = 6–9; **P < 0.01 and ***P < 0.005 versus the WT protein.