Abstract
INTRODUCTION: C-Met plays important roles in treatment resistance, tumor invasion, and metastasis. In this study, we used a small molecule inhibitor of c-Met, crizotinib, in cetuximab-resistant, mutant KRAS-driven colorectal cancer cell lines and assessed radiosensitization. MATERIALS AND METHODS: A tissue microarray containing colorectal tumors was used to study the relationship between KRAS mutations and c-Met expression. For in vivo studies, we used the KRAS mutant cell lines HCT116, DLD1, and LoVo. Colony formation assays were performed to assess the effects of crizotinib and cetuximab. Immunoblot analysis was used to determine the effect of crizotinib on c-Met and downstream pathways and DNA damage response. We then selected noncytotoxic doses of crizotinib to assess clonogenic survival with radiation. To study potential mechanisms of radiosensitization, cell cycle analysis was performed using flow cytometry. RESULTS: Analysis of the tissue microarray revealed that KRAS mutant tumors had active c-Met signaling. KRAS mutant cell lines LoVo, HCT116, and DLD1 were resistant to cetuximab but sensitive to crizotinib. Pretreatment with crizotinib for 24 hours radiosensitized LoVo, DLD1, and HCT116 cell lines with enhancement ratios of 1.54, 1.23, and 1.30, respectively. Immunoblot analysis showed that crizotinib blocked radiation-induced c-Met phosphorylation and attenuated downstream signaling pathways. Cell cycle analysis revealed minimal G1 arrest with crizotinib. Additionally, crizotinib completely blocked HGF induced cell migration. CONCLUSIONS: Inhibition of c-Met with crizotinib effectively sensitizes cetuximab-resistant KRAS mutant colorectal cancer cell lines to radiation. Crizotinib has the potential to improve outcomes in locally advanced rectal cancer patients undergoing chemoradiation.
Introduction
Despite recent advances, colorectal cancer remains a leading cause of cancer-related death [1]. Surgical resection is the primary treatment for this disease; however, recent studies suggest patients having a complete response to neoadjuvant therapy may be candidates for organ preservation [2], [3], [4]. Surgery has several long-term implications including the need for a permanent ostomy in some patients, chronic changes in bowel and bladder habits, and sexual dysfunction. Radiation is commonly used for organ preservation in other diseases involving the pelvis, such as anal, bladder, prostate, and cervix cancers. Unfortunately, for rectal cancer, the complete response rate with chemoradiation is only 10%-15% [5], [6], making few patients eligible for this approach. Several agents have been tested in combination with standard chemoradiation therapy in attempt to improve response rates. EGFR inhibitors including cetuximab have shown efficacy when combined with chemotherapy in KRAS wild-type patients; however, clinical trials examining EGFR inhibitors in combination with chemoradiation therapy have not demonstrated improvement in complete response rates [7] even in patients with KRAS wild-type tumors [8]. There is a great need for developing more effective chemoradiation regimens for this disease.
C-Met is a receptor tyrosine kinase that regulates multiple cancer-related processes. C-Met activation induces an invasive growth program characterized by cell spreading, cell-cell dissociation, motile phenotype acquisition, migration, and proliferation [9], [10], [11]. The main ligand for c-Met is hepatocyte growth factor (HGF). Upon HGF binding, c-Met forms an active dimer that promotes signal transduction directly through its kinase activity and indirectly through the scaffolding protein Gab1 [12]. C-Met can be activated in cancer through several mechanisms, the most common being overexpression at the transcriptional level. In several disease sites, high expression of c-Met is a poor prognostic factor [13]. In colorectal cancer, high c-Met expression has been associated with an increased risk of metastatic disease and decreased survival in patients, including patients with early-stage disease [14], [15], [16].
In this study, we examined the radiosensitizing potential of crizotinib. Crizotinib is a small molecule inhibitor that is FDA approved for advanced-stage non–small cell lung cancer expressing the EML4-ALK fusion protein or ROS1. Crizotinib is a potent c-Met inhibitor with a lower IC50 (11 nM) for this protein than for ALK (24 nM) [17]. Given the important role c-MET plays in colorectal cancer, we hypothesized that targeting this kinase would enhance the cytotoxic effects of radiation and alter radiation-induced cellular processes such as cell migration.
Methods
Cell Culture
The colorectal cancer cell lines HCT116, DLD1, LoVo, SW48, HT29, RKO, CaCo2, LS411N, and SW620 were obtained from ATCC and maintained in their recommended media (DMEM or F-12) supplemented with 10% fetal bovine serum (Life Technologies) and penicillin/streptomycin. Crizotnib was obtained from Sigma-Aldrich and was dissolved in dimethyl sulfoxide and stored in aliquots at −20°C.
Radiation Technique
Radiation was delivered using a Philips RT250 orthovoltage unit (Kimtron Medical) at a dose rate of approximately 2 Gy/min. Dosimetry was carried out using an ionization chamber connected to an electrometer system directly traceable to a National Institute of Standards and Technology calibration.
Clonogenic Survival Assays
Clonogenic survival assays were performed as previously described [18]. Crizotinib was added to cell culture plates 1 hour or 24 hours prior to radiation with 0 to 8 Gy. Depending on the assay, crizotinib was then removed from the plates 4 hours after radiation or left on until the end of the experiment. Cells were incubated until visible colonies were present. Colonies were fixed with methanol/acetic acid (7:1) and stained with crystal violet. The number of colonies containing ≥50 cells was determined. Enhancement ratios were calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose with drug treatment.
Immunoblotting
Whole cell lysates were prepared with SDS lysis buffer (10 mM Tris, 2% SDS) supplemented with phosphatase inhibitor (Thermo Scientific# 78420) and a protease inhibitor (Sigma# P8340). Antibodies for EGFR (Santa Cruz #SC-03), phospho-EGFR (CST #4407S), ERK (CST #9107S), phospho-ERK (CST #4376S), Met (CST #3127S), phospho-Met (CST #3077S), AKT (CST #9272S), phospho-AKT (CST #4060S), γH2AX (Millipore #05-636), and GAPDH (CST #2118S) were used with the appropriate HRP-labeled secondary antibodies (CST #7074S and 7076S). The density of band was quantified using ImageJ (Version 1.52 g) software, and the relative expression was determined after normalizing to cells that express highest levels and shown below the blot.
Tissue Microarray
A tissue microarray containing 65 surgical specimens from patients with colorectal cancer was analyzed for protein expression and KRAS mutational status. DNA was extracted from each core, and polymerase chain reaction was used to determine KRAS mutational status. Immunohistochemistry was then performed for c-Met, phospho-Met, and EGFR. Each core was scored by a single pathologist using the Allred system.
Statistics
The mean, median, and standard error were calculated using Microsoft Excel software. A Student's t test (paired when applicable) was used to compare treatment groups. All experiments were performed in at least triplicate to ensure reproducibility. For the tissue microarray, we used the Wilcoxon rank sum test to compare immunohistochemistry scores between KRAS mutant and KRAS wild-type tumors.
Results
c-Met as a Therapeutic Target in KRAS Mutant Colorectal Cancer
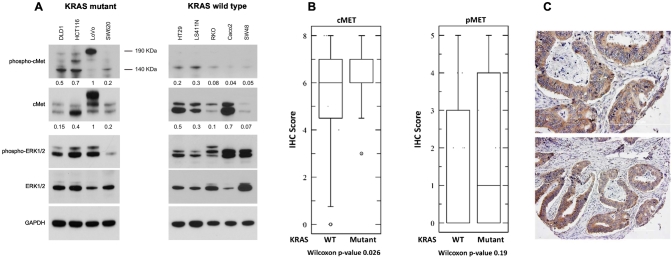
In order to determine the potential of c-Met as a molecular target for colorectal cancer, we analyzed expression patterns of this protein and related pathways in colorectal cancer cell lines, and a colorectal tissue microarray. Nine colorectal cancer cell lines with a range of mutations (Table 1) were cultured in standard media with serum. Once cells were 80%-90% confluent, protein was isolated for Western blot analysis. Basal expression of c-Met, p-Met, ERK, and pERK was determined. Several of the cell lines had evidence of an active c-Met pathway, with high basal expression of c-Met, p-Met, and pERK (Figure 1A). All cell lines with a KRAS mutation has detectable levels of phosphorylated c-Met. LoVo, DLD1, and HCT116 had the highest levels of basal pMET out of the nine cell lines tested.
Table 1.
Colorectal Cancer Cell Line Panel
| Cell Line | Mutations |
|---|---|
| LoVo | KRAS heterozygous G13D |
| FBXW7 heterozygous R505C | |
| SW48 | EGFR G719S |
| FBXW7 S668fs* (Frame_Shift_Del) | |
| AKT2, FANCD2, FGFR1 | |
| HT29 | BRAF (V600E, T119S) |
| PI3CA | |
| FGFR3 | |
| RKO | BRAF |
| PI3CA | |
| BUB1 (Y259C) | |
| DNMT3B (V616M) | |
| BRAF (V600E) | |
| TGFBR2 (V561A) | |
| HSP90AA1 (D515N, N626D) | |
| Caco2 | P53 |
| LS411N | BRAF homozygous V600E |
| FBXW7 heterozygous P505H | |
| EGFR (E34*, Q478E, T940A) | |
| ErbB3, ErbB4 (P837G), DAPK (S1096N), | |
| STAT3 (T178fs), Src (V380M) | |
| HIP1, HIPK3, HDAC3, JAK2 | |
| cMET (T861) | |
| DLD1 | KRAS G13D |
| P53 | |
| PIK3CA E545K and D549N | |
| HCT116 | KRAS G13D |
| PIK3CA H1047R | |
| cMet V237fs (Frame Shift) | |
| FGFR1 and FGFR2 | |
| SW620 | KRAS G12V |
| HCT116 | KRAS G13D/+ (heterozygous) |
Figure 1.
C-Met signaling in KRAS mutant colorectal cancer. A panel of colorectal cancer cells lines with various mutations (see Table 1) was used to study baseline levels of c-Met and related proteins. Western blot analysis was performed for c-Met and related proteins, and the densities of the bands were quantified (A). Many of the cell lines, including each of the KRAS mutant cell lines, had active c-Met signaling. We then analyzed a tissue microarray containing untreated colorectal cancers. KRAS mutational status was determined by PCR. Immunohistochemistry for c-Met and p-Met was performed and quantified using the Allred system. KRAS mutant tumors had high expression of c-Met and p-Met. (B) The median and 25-75 percentile range for each protein marker. (C) Representative tumor cores showing c-Met staining are shown.
To investigate the potential relationship between KRAS mutations and c-Met expression, we analyzed a tissue microarray containing 65 surgical specimens from untreated colorectal cancer patients. KRAS mutational status was able to be determined using the polymerase chain reaction (PCR) for 54 tumors. Twenty-seven colorectal tumors had a KRAS mutation, and 27 tumors were KRAS wild type. We performed immunohistochemistry using antibodies targeting c-Met, p-Met, and EGFR. C-Met signaling was highly active in several of the KRAS mutant tumors. Box plots displaying the median and 25 to 75 percentile are shown in Figure 1B. Representative cores with cMET staining are shown in Figure 1C. The mean Allred score for c-Met was 6.7 (95% CI 5.8-7.5) in KRAS mutant tumors compared to 5.2 (95% CI 4.4-6.0; P = .026) in KRAS wild-type tumors. The mean Allred score for phospho-Met was 1.9 (95% CI 1.1-2.8) in KRAS mutant tumors compared to 1.4 (95% CI 0.6-2.2; P = .19) in KRAS wild-type tumors. Additionally, the mean Allred score for EGFR was 4.9 (95% CI 3.6-6.1) in KRAS mutant tumors compared to 2.3 (95% CI 1.1-3.5; P = .006) in KRAS wild-type tumors.
Targeting c-MET in Cetuximab-Resistant KRAS Mutant Colorectal Cancer
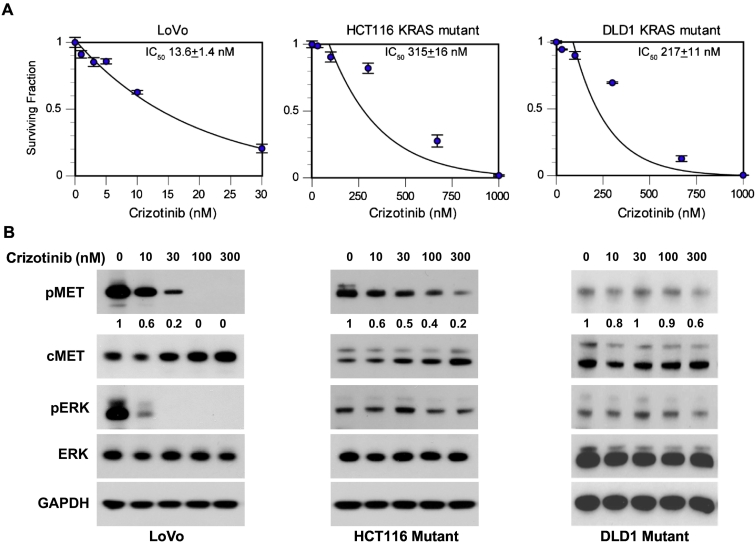
KRAS mutations confer resistance to anti-EGFR therapies in colorectal cancer. Given the important role of c-Met and related downstream signal transduction pathways in colorectal cancer, we tested the effectiveness of the c-Met small molecule inhibitor crizotinib in KRAS mutant cell lines. First, we confirmed that our panel of KRAS mutant cell lines was resistant to cetuximab with a colony formation assay. As expected, the KRAS mutant cell lines LoVo, HCT116, and DLD1 were resistant to cetuximab (surviving fraction >95% at 100 μg/ml, Supplemental Figure 1). We then performed a series of clonogenic survival assays [18] using crizotinib in our panel of KRAS mutant colorectal cancer cell lines. Each of our cell lines was sensitive to crizotinib with IC50 values of 13.6 ± 1.4 nM for LoVo, 315 ± 16 nM for HCT166, and 217 ± 11 nM for DLD1 (Figure 2A). To confirm inhibition of c-Met, we isolated protein from our cell lines 24 hours after treatment with crizotinib and performed Western blot analysis. Crizotinib attenuated phosphorylation of c-Met in all three cell lines (Figure 2B). In LoVo, c-Met phosphorylation was reduced following treatment with 10-30 nM crizotinib corresponding to the IC50 for this cell line. Additionally, HCT116 and DLD1 showed decreased c-Met phosphorylation in the 100- to 300-nM range which was consistent with their IC50 in the clonogenic survival assays. We also saw an effect on targets downstream from c-Met and KRAS, including decreased ERK activity in crizotinib treated LoVo and HCT116 cell lines.
Figure 2.
Effects of crizotinib in colorectal cancer cell lines. Three KRAS mutant colorectal cancer cell lines were used for a clonogenic survival assay with the c-Met inhibitor crizotinib. (A) Each cell line was sensitive to the drug alone. Whole cell lysates from crizotinib-treated cells were used for Western blot analysis. (B) Crizotinib decreased p-Met levels and altered downstream signal transduction pathways. The density of each pMET band is displayed below the blot; values were normalized to untreated cells.
Effect of Radiation on c-Met Phosphorylation in KRAS Mutant Colorectal Cancer
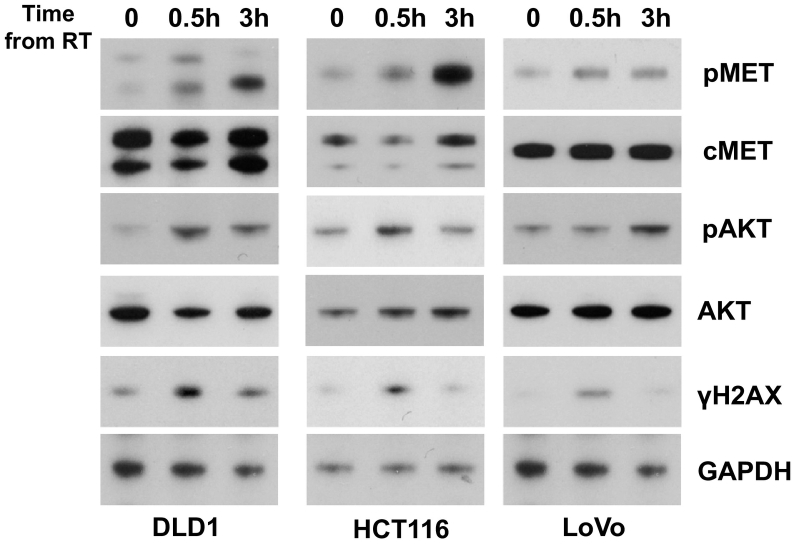
To study the therapeutic potential of c-Met inhibition and radiation therapy in colorectal cancer, we selected three KRAS mutant, cetuximab-resistant cell lines and examined the effect of radiation therapy on c-MET phosphorylation. DLD1, HCT116, and LoVo cell lines were irradiated then harvested at 0.5 and 3 hours. Treatment with radiation therapy induced c-Met phosphorylation within 3 hours in all cells lines (Figure 3).
Figure 3.
Effects of radiation on c-Met activation. Three KRAS mutant colorectal cancer cell lines were treated with radiation (10 Gy). Whole cell lysates were obtained at 30 minutes and 3 hours following radiation. Radiation induced Met phosphorylation in each cell line tested. AKT phosphorylation also increased following radiation therapy. The conditions used were adequate to induce DNA double-strand breaks as indicated by ɣH2AX staining.
These studies suggest that radiation induces activation of c-Met in KRAS mutant CRC cell lines. Given the important role c-Met plays in cell migration, we examined the effects of c-Met stimulation with radiation therapy on cell migration using a wound closure assay. Treatment with radiation enhanced cell migration in all three cell lines (Supplemental Figure 2).
Effect of Crizotinib on Radiation Response
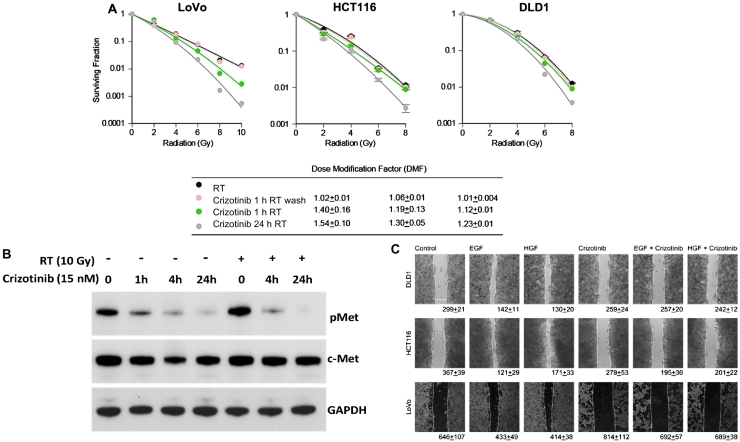
After determining the response to crizotinib in our panel of KRAS mutant CRC cell lines, we next performed a series of clonogenic survival assays to study the radiosensitizing potential of this drug. Cell lines were plated at clonogenic density then treated with the following schedules of crizotinib: 24 hours prior to radiation, 1 hour prior to radiation and 4 hours after, and continuous exposure starting at 24 hours prior to radiation and continued for the duration of the study. Once colonies formed, plates were quantified and enhancement ratios calculated. Pretreatment with crizotinib for 24 hours radiosensitized LoVo, DLD1, and HCT116 cell lines with enhancement ratios of 1.54 ± 0.10, 1.23 ± 0.01, and 1.30 ± 0.05, respectively (Figure 4A). Interestingly, treatment with crizotinib for 1 hour prior to radiation resulted in only modest dose enhancement, suggesting that prolonged exposure to crizotinib (24 hours) is required for optimal radiosensitization.
Figure 4.
Radiosensitization with crizotinib. The effects of various schedules of crizotinib on irradiated colorectal cancer cell lines were assessed using a clonogenic survival assay. Cell lines were treated for 24 hours or 1 hour with crizotinib prior to radiation; media were changed at 4 hours (wash) or left on for the duration of the experiment. (A) Noncytotoxic doses of crizotinib sensitized each cell line to radiation therapy. The greatest sensitization was seen with prolonged (24 hours) crizotinib exposure prior to radiation. (B) Western blot analysis of LoVo cells treated with radiation and crizotinib demonstrated that this drug blocked radiation-induced Met phosphorylation. We also performed a cell migration assay which found that crizotinib blocked HGF- and EGF-induced cell migration.
To better understand the mechanism of radiosensitization with crizotinib, we performed Western blot analysis in cell lines treated with crizotinib and radiation. We found that treatment with radiation alone increased phosphorylation of c-Met and that radiation-induced activation of c-Met was effectively blocked by crizotinib (Figure 4B). These results suggest that c-Met plays a role in the radiation response and targeting c-Met with Crizotinib blocks the cellular response to radiation therapy.
To further study the effect of c-MET inhibition with crizotinib in KRAS mutant colorectal cancer cell lines, we performed a series of functional assays. Treatment with HGF or EGF induced wound closure in all cell lines tested. Given c-Met's important role in cell migration and invasion, we hypothesized that crizotinib could alter this process. Cells were plated and grown to 80% confluency prior to wound creation. We then treated cells with crizotinib. Crizotinib effectively blocked HGF-induced and EGF-induced cell migration (Figure 4C).
Mechanism of Radiosensitization
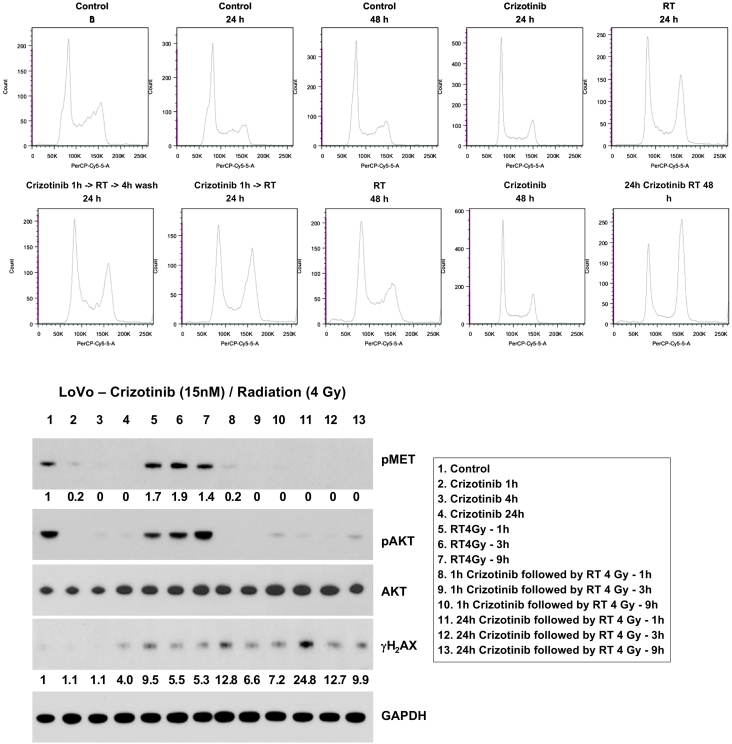
To better characterize the mechanism behind radiosensitization with crizotinib, we analyzed the effects of this drug and radiation on the cell cycle and DNA double-strand break repair. Cell cycle analysis using propidium iodide and flow cytometry analysis revealed minimal G1 or G2 arrest with crizotinib alone (Figure 5, A-B). However, crizotinib prolonged G2 arrest in irradiated DLD cell lines 24 hours after radiation, most likely related to increased/persistent DNA damage. Overall, there were minimal effects on cell cycle in the cell lines tested. Western blot was performed with anti-γH2AX antibodies to study the effect of crizotinib on DNA double-strand breaks. Treatment with crizotinib resulted in more DNA double-strand breaks, as indicated by increased yH2AX staining, shortly after radiation therapy, and this effect persisted at 3 and 9 hours after treatment (Figure 5C). Additionally, prosurvival downstream radiation-induced AKT activation was attenuated with crizotinib treatment.
Figure 5.
In order to assess the mechanism of radiosensitization with crizotinib, we analyzed cell cycle and DNA damage repair following treatment with crizotinib and/or radiation. (A) Crizotinib alone had minimal effect on the cell cycle. However, a prolonged G2 arrest was seen in cell lines treated with crizotinib and radiation likely related to increased DNA damage. (B) Western blot analysis of the DNA double-strand break maker γH2AX showed increased radiation-induced DNA damage in crizotinib-treated cell lines and prolonged DNA double-strand break repair. (B) Additionally, radiation-induced AKT activation was attenuated with crizotinib. The density of each band is displayed below the lane.
Discussion
In this study, we identified c-Met as a potential molecular target for radiosensitization in KRAS mutant colorectal cancer. We selected a panel of colorectal cancer cell lines with varying mutations associated with resistance to targeted therapies including cetuximab. The KRAS mutant cell lines we tested were resistant to EGFR targeted therapy but were sensitive to the c-Met inhibitor crizotinib with IC50 values in the nanomolar range. Treatment with radiation therapy induced c-Met phosphorylation and triggered a number of cellular processes including cell migration. The c-Met inhibitor crizotinib effectively blocked radiation-induced c-Met activation and altered downstream signal transduction pathways involving ERK and AKT. Crizotinib treatment was also associated with increased DNA damage after radiation therapy and delayed resolution of DNA double strand breaks.
Cetuximab is an EGFR targeted therapeutic antibody that has efficacy in patients with KRAS wild-type tumors. Cetuximab has been studied as a potential radiation sensitizer in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiation. In a randomized phase II study by US Oncology, the addition of cetuximab to 5-FU–based chemoradiation had no effect on complete response rates or recurrence-free survival, and its use was associated with increased toxicity [7]. The Expert-C study examined the addition of cetuximab to capecitabine and oxaliplatin with radiation therapy. This randomized phase II study found no benefit in the entire study population; however, in the subset of patients whose tumors were KRAS and BRAF wild type, the addition of cetuximab was associated with improved radiographical response and overall survival [8]. In order to make nonoperative management a viable option for patients with rectal cancer, we need better radiosensitizing therapies. Selecting specific therapies for patients based off the biology of their disease has the potential to optimize the efficacy of novel drug-chemoradiation combinations.
Most radiation sensitizers affect DNA repair and/or alter cell cycle kinetics [19], [20]. In this study, we examined these cellular processes in response to crizotinib treatment. Crizotinib alone had minimal effect on the cell cycle. G1 arrest is common with many kinase inhibitors including cetuximab, and the presence of a G1 arrest has been hypothesized to be antagonistic to 5-FU and radiation combinations [21], [22], [23]. Therefore, drugs and dosing schedules that minimize G1 arrest are ideal to combine with existing chemoradiation regimens.
In our study, crizotinib had a substantial effect on DNA damage repair with an increased amount of DNA double-strand breaks shortly after radiation and delayed resolution of DNA double-strand breaks. C-Met signaling and its induction by ionizing radiation have been shown to be associated with radiation resistance. The mechanism behind this phenomenon has not been fully elucidated. Work by DeBacco et al. has demonstrated that MET-induced signal transduction pathways promote ATM hyperactivation in glioblastoma models [24]. Other reports have suggested that targeting c-Met leads to increased DNA damage following radiation therapy and attenuates DNA double-strand break repair [25]. Earlier work has shown that HGF protects cells from DNA damage ([26] Fan) and that blocking HGF:c-Met interaction can reverse this phenomenon. Additionally, c-Met inhibition has been shown to inhibit homologous recombination [25]. In our study, we found that treatment with crizotinib resulted in increased number of DNA double-strand breaks shortly after radiation therapy in KRAS mutant colorectal cancer cell lines. Additionally, prosurvival signal transduction pathways involving ERK and AKT were activated following radiation, and crizotinib attenuated this response.
Prior studies have examined c-Met as a potential target for radiosensitization. Buchanan et al. studied the anti-HGF antibody AMG-102 in glioblastoma models [27]. They found increased DNA damage and increased mitotic catastrophe with HGF neutralization. Additionally, in subcutaneous xenograft models, they demonstrated a synergistic effect with radiation. Bhardwaj et al. studied the c-Met inhibitor MK-8003 in a non–small cell lung cancer model. This study identified increased expression of c-MET protein shortly after radiation. MK-8003 sensitized cell lines with high c-Met expression but not cell lines with low c-Met expression [28].
As KRAS mutations confer resistance to many targeted therapies including EGFR inhibitors, testing c-Met inhibitors in this population is a potential strategy to improve the effectiveness of chemoradiation. Future studies using KRAS status to select an appropriate agent for radiosensitization is an attractive strategy. For example, patients with KRAS wild-type tumors could receive an EGFR inhibitor, and patients with a KRAS mutation could receive a c-Met inhibitor with standard chemoradiation.
Conclusions
This report demonstrates the radiosensitizing potential of the c-Met inhibitor crizotinib in KRAS mutant colorectal cancer. Our tissue microarray analysis suggests that nonmetastatic KRAS mutant colorectal cancers have high protein expression of c-Met. By targeting this kinase, we potentially can radiosensitize tumors that are resistant to EGFR targeted therapies.
Footnotes
Funding: The work was supported by the University of Michigan Gastrointestinal SPORE grant P50CA130810.
Conflicts of interest statement: The authors have no conflicts of interest to report.
Presented at the American Association for Cancer Research Annual Meeting 2017, Washington DC.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2018.10.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174–183. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 3.Appelt AL, Ploen J, Harling H, Jensen FS, Jensen LH, Jorgensen JC, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. doi: 10.1016/S1470-2045(15)00120-5. [DOI] [PubMed] [Google Scholar]
- 4.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Jr., Silva e Sousa AH, Jr., Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–717. doi: 10.1097/01.sla.0000141194.27992.32. [discussion 17-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 6.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 7.McCollum AD, Kocs DM, Chadha P, Monticelli MA, Boyd TE, Fain JD, Kasper M, Sanchez J, Simon M. Randomized phase II trial of preoperative chemoradiotherapy with or without cetuximab in locally advanced rectal adenocarcinoma. J Clin Oncol. 2004;32(Suppl. 3) [abstr 537] [Google Scholar]
- 8.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30(14):1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 9.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2(4):289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 10.Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27(1):85–94. doi: 10.1007/s10555-007-9107-6. [DOI] [PubMed] [Google Scholar]
- 11.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9(6):314–326. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 12.Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149(7):1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3(1 Suppl):S21–S35. doi: 10.1177/1758834011422557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginty F, Adak S, Can A, Gerdes M, Larsen M, Cline H, Filkins R, Pang Z, Li Q, Montalto MC. The relative distribution of membranous and cytoplasmic met is a prognostic indicator in stage I and II colon cancer. Clin Cancer Res. 2008;14:3814–3822. doi: 10.1158/1078-0432.CCR-08-0180. [DOI] [PubMed] [Google Scholar]
- 15.Liu Yan, Yu Xiao-Feng, Zou Jian, Luo Zi-Hua. Prognostic value of c-Met in colorectal cancer: a meta-analysis. World J Gastroenterol. 2015;21(12):3706–3710. doi: 10.3748/wjg.v21.i12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan SA, Forslund A, Nash GM, Gimbel M, Yamaguchi Y, Culliford AT. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui JJ, Tran-Dube M, Shen H, Nambu M, Kung PP, Pairish M, Jia L, Meng J, Funk L, Botrous I. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J Med Chem. 2011;54(18):6342–6363. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. Int J Radiat Oncol Biol Phys. 1988;15:953–958. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence TS. Radiation sensitizers and targeted therapies. Oncology (Williston Park) 2003;17(12 Suppl. 13):23–28. [PubMed] [Google Scholar]
- 20.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13(1):13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 21.Cuneo KC, Nyati MK, Ray D, Lawrence TS. EGFR targeted therapies and radiation: optimizing efficacy by appropriate drug scheduling and patient selection. Pharmacol Ther. 2015;154:67–77. doi: 10.1016/j.pharmthera.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng DD, Fan ZZ, Lu YY, DeBlasio TT, Scher HH, Mendelsohn JJ. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–3669. [PubMed] [Google Scholar]
- 23.Di Gennaro EE, Barbarino MM, Bruzzese FF, De Lorenzo SS, Caraglia MM, Abbruzzese AA, Comella P, Caponigro F, Pepe S, Budillon A. Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 (‘Iressa’), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cells. J Cell Physiol. 2003;195:139–150. doi: 10.1002/jcp.10239. [DOI] [PubMed] [Google Scholar]
- 24.De Bacco F, D'Ambrosio A, Casanova E, Orzan F, Neggia R, Albano R, Verginelli F, Cominelli M, Poliani PL, Luraghi P. MET inhibition overcomes radiation resistance of glioblastoma stem-like cells. EMBO Mol Med. 2016;8(5):550–568. doi: 10.15252/emmm.201505890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medova M, Aebersold DM, Zimmer Y. MET inhibition in tumor cells by PHA665752 impairs homologous recombination repair of DNA double strand breaks. Int J Cancer. 2012;130(3):728–734. doi: 10.1002/ijc.26058. [DOI] [PubMed] [Google Scholar]
- 26.Fan S, Ma YX, Gao M, Yuan RQ, Meng Q, Goldberg ID, Rosen EM. The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair. Mol Cell Biol. 2001;21(15):4968–4984. doi: 10.1128/MCB.21.15.4968-4984.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchanan IM, Scott T, Tandle AT, Burgan WE, Burgess TL, Tofilon PJ, Camphausen K. Radiosensitization of glioma cells by modulation of Met signalling with the hepatocyte growth factor neutralizing antibody, AMG102. J Cell Mol Med. 2011;15(9):1999–2006. doi: 10.1111/j.1582-4934.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhardwaj V, Zhan Y, Cortez MA, Ang KK, Molkentine D, Munshi A, Raju U, Komaki R, Heymach JV, Welsh J. C-Met inhibitor MK-8003 radiosensitizes c-Met-expressing non-small-cell lung cancer cells with radiation-induced c-Met-expression. J Thorac Oncol. 2012;7(8):1211–1217. doi: 10.1097/JTO.0b013e318257cc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material