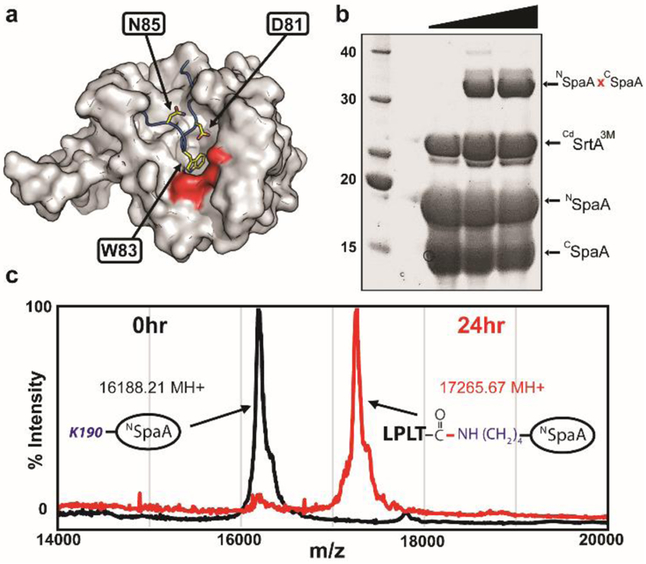
Figure 1.
Mutationally activated CdSrtA catalyzes lysine-isopeptide bond formation. (A) The structure of CdSrtAWT harbors an inhibitory “lid” structure (blue). Side chains that were mutated to activate the enzyme are shown as yellow sticks. The surface of the catalytic site is colored red. (B) Protein-protein ligation using the activated CdSrtA3M enzyme. SDS-PAGE analysis of the reaction demonstrating formation of the lysine-isopeptide linked NSpaAxCSpaA product. The reaction (100μM enzyme, 300μM CSpaA and NSpaA) was sampled at 0, 24 and 48 hours. (C) High yield protein-peptide labeling with CdSrtA3M. MALDI-MS data showing that >95% NSpaA is labeled with peptide containing the sort-tag, LPLTGpeptide. MALDI-MS spectra recorded at 0 (black) and 24h (red) are overlaid.