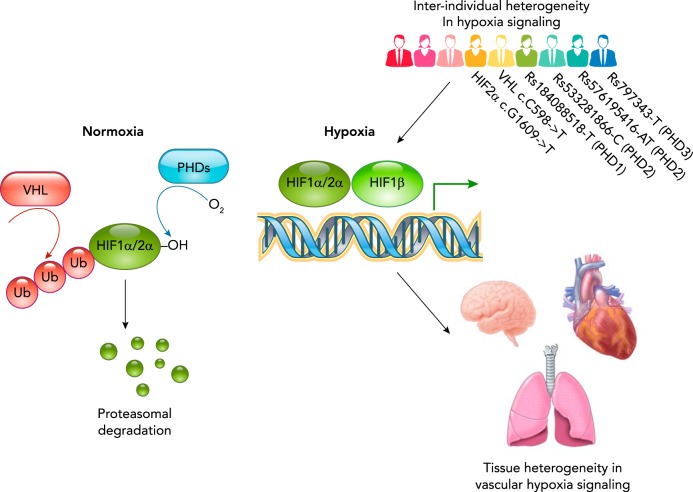
FIGURE 1.
Heterogeneity of hypoxia signaling
Under normal oxygen levels, HIF-1α and HIF-2α are hydroxylated on conserved prolyl residues by a set of oxygen-dependent prolyl hydroxylases (PHD1–3). This results in the recognition of HIF-1α and HIF-2α by the von Hippel-Lindau protein, leading to ubiquitination and subsequent proteasomal degradation. In response to lower oxygen levels, PHD enzymes will be inhibited, allowing for the accumulation of HIF-1α and HIF-2α. They subsequently form a complex with constitutively expressed HIF-1β and induce the expression of target genes. These genes are involved both in systemic responses such as increased angiogenesis and in changes of cellular energy metabolism. There is considerable heterogeneity in the hypoxic response between individuals, between different vascular beds, and even between individual endothelial cells. Understanding this complexity and heterogeneity is a prerequisite for designing targeted therapies that minimize unintended collateral effects.