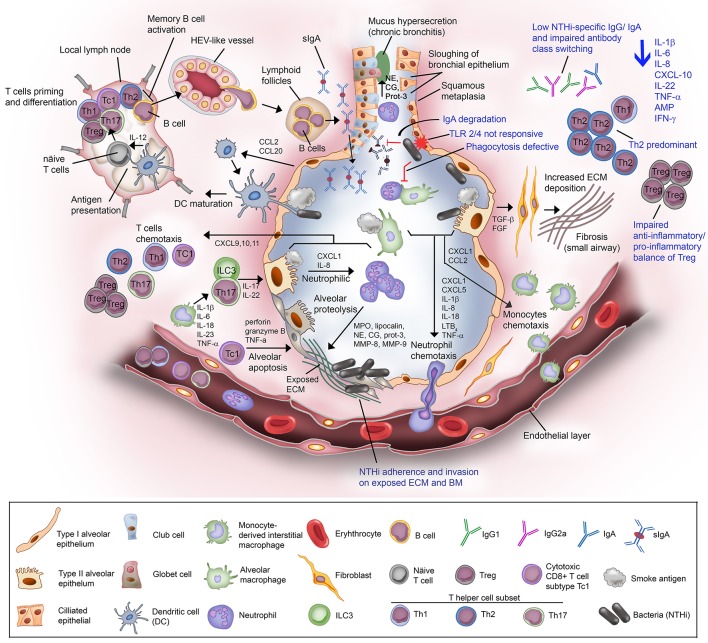
Figure 2.
Non-typeable H. influenzae-dependent immune responses in the lower airway of COPD patients result in inflammation. Airway epithelium exposed to cigarette or tobacco smoke display an increased permeability with compromised tight junctions, and airway remodeling (goblet cell hyperplasia and small airway squamous metaplasia). Cigarette smoke causes the activation of airway epithelium and alveolar macrophages. The activated airway structural and resident immune cells release an array of chemotactic factors responsible for recruitment of inflammatory and immune cells to the lung. Activated epithelium produces TGF-β and FGF that triggers the production of ECM molecules by fibroblasts. Increased deposition of ECM causes progression of fibrosis and air flow limitation. The chemokines CXCL1 and IL-8, and LTB4 attract the circulating neutrophils through the receptors CXCR2 and BLT1, respectively. Meanwhile, CXCL1 and CCL2 targeting the receptors CXCR2 and CCR2 on blood monocytes are also released. Recruited blood monocytes differentiate into macrophages in the airway tissue. Activated alveolar macrophage and epithelium cell also release inflammasome (1L-1β and IL-18) for neutrophils survival and activation of helper T cells Th17. The chemokine IL-23 are released by macrophages to attract T helper cell subset Th17, and ILC3. Both Th17 and ILC3 will release IL-17 and IL-22 that will act on the alveolar epithelium to release CXCL1 and IL-8 for enhanced recruitment of neutrophils, resulting in neutrophilic inflammation. Activated neutrophils are thereafter degranulated and release myeloperoxidase (MPO), lipocalin, neutrophil elastase (NE), cathepsin-G (CG), proteinase-3 (Prot-3), and matrix metalloprotease (MMP) 8 and 9. The granulated products are proteolytic and elastilolytic to aveolar, causing alveolar destruction and emphysema. In addition, NE, CG, and Prot-3 are also targeting goblet cells and submucosal glands to induce hypersecretion of mucus. Dendritic cells carrying the receptors CCR2 and CCR6 are recruited to airway tissue via chemottractants CCL2 and CCL20. The dendritic cells uptake the antigen (smoke residues), and present the antigens to the naïve T cells at lymph nodes. Uncommitted T lymphocytes are thereafter primed to the presented antigen and activated by IL-12 derived from dendritic cells (professional antigen presenting cells; APC). Mature/activated T cells expressing receptor CXCR3 are chemotactic toward CXCL9, CXCL10, and CXCL11 and are recruited to the lung tissue. Cytotoxic CD8+ T cell subtype Tc1 releases perforin and granzyme B resulting in epithelial apoptosis contributing to emphysema progression. For the humoral immune response, B cells are activated by Th2, enter the circulation via high-endothelial venule (HEV)-like vessel and transported to lung tissue, and organized into lymphoid follicles at peripheral airway. B cell-derived plasma cells from lymphoid follicles release IgA, and secreted into airway lumen as secretory IgA (sIgA) via the polymeric immunoglobulin receptor. Mucosal antibodies play an important role to eradicate pathogens and noxious antigens via immune exclusion. However, the airway defense by sIgA is diminished by NTHi IgA protease that degrade the antibodies. TLR2 and TLR4 of the airway phagocytes and epithelium following exposure to cigarette smoke are not responding to P6 and LOS of NTHi. This results in defective phagocytosis and delayed bacterial clearance from the airway. The suppressed TLR4 induction in T cells has also lead to Th2 predominant immune response, with low production of IFN-γ and reduced T cell-mediated immune killing of NTHi. Moreover, NTHi downregulates Foxp3 of Tregs and thus impairs the anti-inflammatory/pro-inflammatory balance of Tregs. The extensive immunosuppressive activity by Tregs diminishes the response of effector T to NTHi stimulation. Lastly, plasma cells from COPD patients fail to produce NTHi-specific antibodies and compromised immunoglobulin class switching. The impairment of the host immune response in COPD toward NTHi infection are labeled in blue. In total, NTHi infection in COPD lung adversely reduces the production of IL-1β, IL-6, IL-8, CXCL-10, IL-22, TNF-α, antimicrobial peptide (AMP), and IFN-γ. This may explain the inefficient eradication of airway pathogens in COPD patients whereby persistent NTHi infection concomitantly escalates the inflammation and thus exacerbation in COPD.