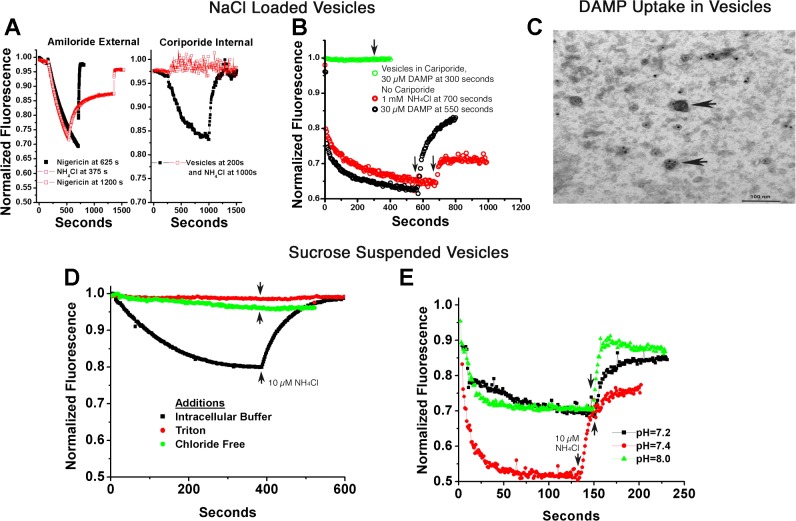
Fig. 4.
Ion gradient-coupled acid transport in human membrane vesicles. A–C: vesicles were reconstituted in 140 mM NaCl and then suspended in 120 mM KCl plus 2 µM acridine orange to study Na+ gradient acidification. A, left: minimal effect of Na/H exchange inhibitors on the outside of inside-out vesicles. Extensive acid uptake occurred (acridine quenching), reversed slowly with 1 mM NH4Cl or rapidly with the H-K ionophore nigericin, 10 µM. The black curve had 100 µM amiloride added with slightly slower quenching, but no claim is made of the relevancy of this small effect. This concentration of amiloride blocked Na/H exchange in recordings of whole cell pH, as reported in Ref. 21. Note that the time course in both curves is much slower for ATP-dependent acid transport in osteoclast membranes (Fig. 3C, black trace). Right: strong inhibition by Na/H exchange inhibitors on the inside of inside-out vesicles. In contrast, reconstitution of the vesicles in 1 mM cariporide, that is, with the inhibitor inside, stops vesicle acidification and acridine orange quenching. The strong dependence of the acidification on the Na+ gradient inside to outside and the requirement for the inhibitor on the same side as the Na+ confirms that acidifying vesicles were reconstituted with the extracellular face of the cell on the inside of the vesicles. This is consistent with Na+/H+ exchanger (NHE) inhibitors being on the same side as the sodium substrate. B: interaction of the weak base DAMP (N-(3-[(2,4-dinitrophenyl)-amino]propyl)-N-(3-aminopropyl)methylamine), with acidified vesicles. Both ammonium chloride (10 mM) and DAMP (30 µM) reduced vesicle acidification, as shown by acridine orange quenching. However, when acidification was blocked by 1 mM intravesicular cariporide, the addition of DAMP had no effect on acridine fluorescence, indicating and the DAMP weak base had no detectible interaction with non-acid vesicles. C: DAMP labeling of acid vesicles. Samples of acid vesicles were treated with DAMP, fixed, and then DAMP was antibody-localized, as described in methods, so that DAMP in vesicles could be visualized by electron microscopy (2, 26). In brief, acid vesicles were reacted with 30 µM DAMP as in B and fixed in glutaraldehyde 1%. Vesicles were recovered by centrifugation and rinsed with goat anti-dinitrophenol, and then horseradish peroxidase anti-goat antibody to make a dense precipitate. The preparation was postlabeled with 20 nM gold antialkaline phosphatase. Vesicles with and without alkaline phosphatase labeling are acidified as shown by election-dense material in random vesicles (arrows); ATP was not added. D: chloride gradient-dependent acid transport. Vesicles reconstituted in intracellular buffer (120 mM KCl) and assayed in isotonic sucrose acidify, and acid uptake is reversed with ammonium chloride (bottom trace). Detergent disruption (top trace) abolishes quenching. A representative trace is also shown with replacement of KCl by potassium gluconate (second trace from top), which eliminated all but trace quenching, consistent with a Cl−-dependent process (see discussion). E: transport at varying extravesicular pH is similar to pH 8. Vesicles reconstituted in intracellular KCl buffer and assayed in isotonic sucrose with 10 mM HEPES adjusted to pH 7.2, 7.4, and 8 acidified similarly, indicating that the process works at slightly alkaline pH. This is important relative to mineral deposition as shown in Fig. 1).