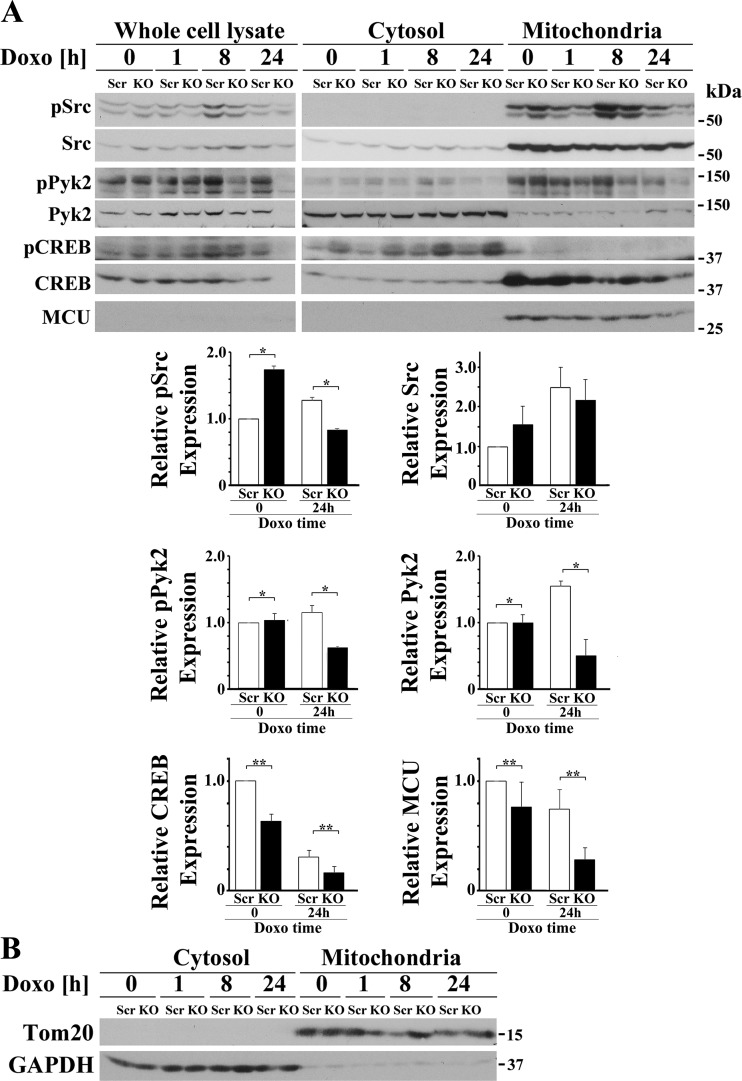
Fig. 6.
Depletion of TRPM2 reduces mitochondrial phosphorylation of Src and Pyk2 and mitochondrial expression of Pyk2, CREB, and MCU. A: TRPM2-depleted and scrambled SH-SY5Y cells were separated into cytosol and mitochondrial fractions and Src, Pyk2, and CREB phosphorylation and expression examined. In both whole cell lysates and mitochondria, phosphorylation of Src and Pyk2 was decreased after doxorubicin treatment of TRPM2-depleted cells. Mitochondrial Pyk2 was also decreased after doxorubicin application. Levels of CREB and the mitochondrial calcium uniporter MCU were reduced in the mitochondrial fraction of KO cells. Similar results were observed in three experiments and representative blots are shown. Densitometry measurements of mitochondrial protein for three experiments were standardized to results for each experiment’s scrambled mitochondrial control at time 0, and the means ± SE of phosphorylated or total Src, Pyk2, CREB, or MCU calculated from three experiments are shown. **P ≤ 0.04, group effect or *P < 0.03, group × doxorubicin exposure time interaction effect analyzed with two-way ANOVA. B: Western blots of Tom20 and GAPDH were done as controls for quality of separation. CREB, cAMP-responsive element-binding protein; Doxo, doxorubicin; KO, knockout; MCU, mitochondrial calcium uniporter; p, phosphorylated; Pyk2, proline-rich tyrosine kinase 2; TRPM2, transient receptor potential melastatin channel subfamily member 2.