Fig. 6.
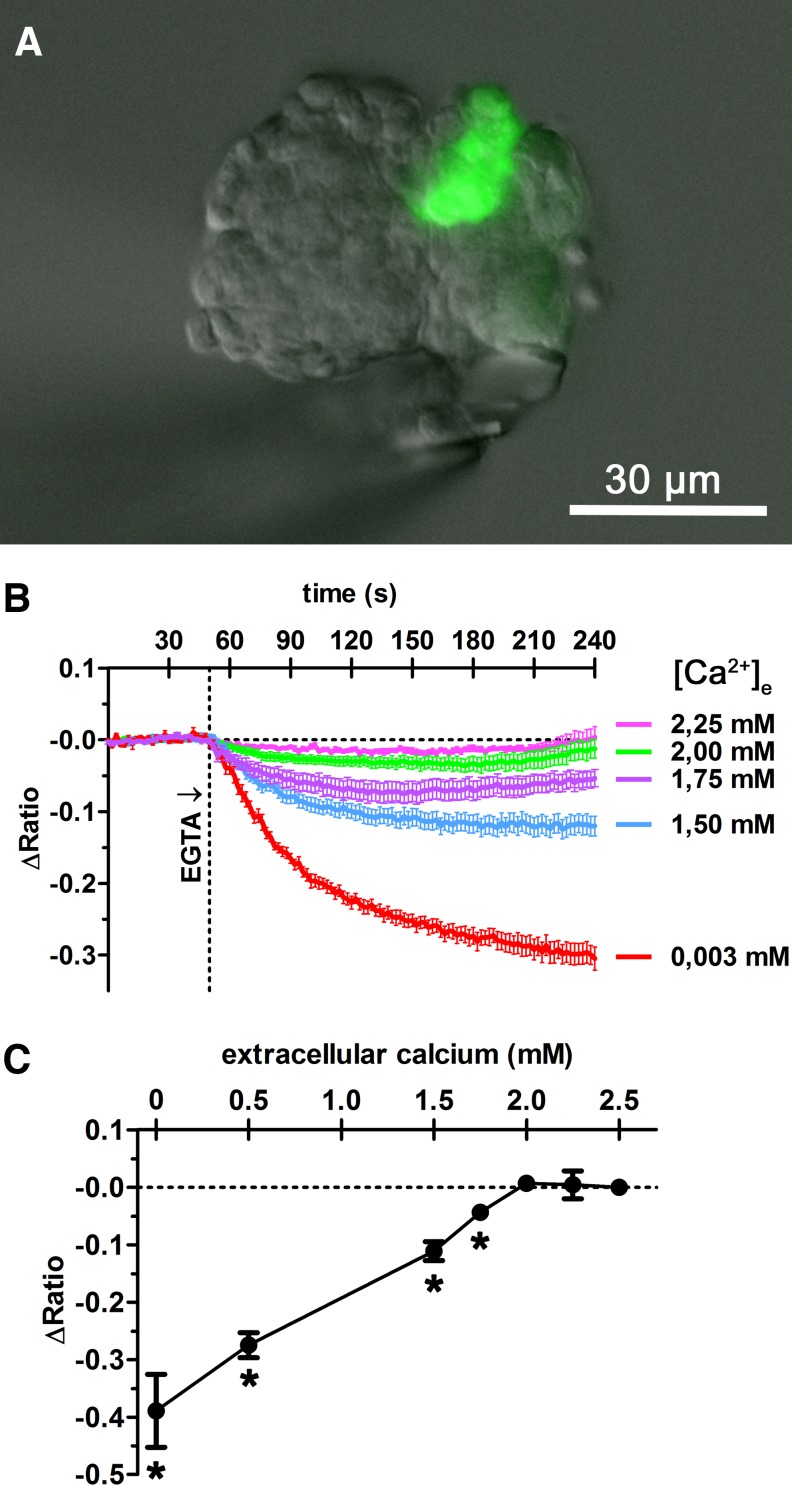
For intracellular calcium measurement, an isolated glomerulus was placed in a perfusion bath and perfused at constant flow of 3 ml/min. To lower the extracellular calcium concentration, different concentrations of EGTA were added to the perfusion medium. The renin-producing cells could be identified by green GFP fluorescence at 63× magnification (A). Fura-2 calcium measurements were performed by placing regions of interest (ROI) on renin-producing cells. Fura-2 emission intensity at 510 nm was detected in response to excitation at 340 nm (excitation maximum of Ca2+-bound state) and 380 nm (excitation maximum of Ca2+-unbound state), providing the ratio (340/380). ΔRatio describes the ratio deviation from average baseline ratio measured in the unstimulated cells. Lowering of the extracellular calcium concentration [Ca2+]e from 2.5 to 2.25 or 2 mM led to transient decreases of intracellular calcium concentration, which recovered to basal values within 4 min (B). Further decreasing extracellular calcium concentration led to a concentration-dependent decrease of cytosolic calcium steady-state levels, measured after 15 min (B, C). *Significant difference from control values (2.5 mM EGTA), P < 0.05; data are means ± SE of 7 renin-producing cells.