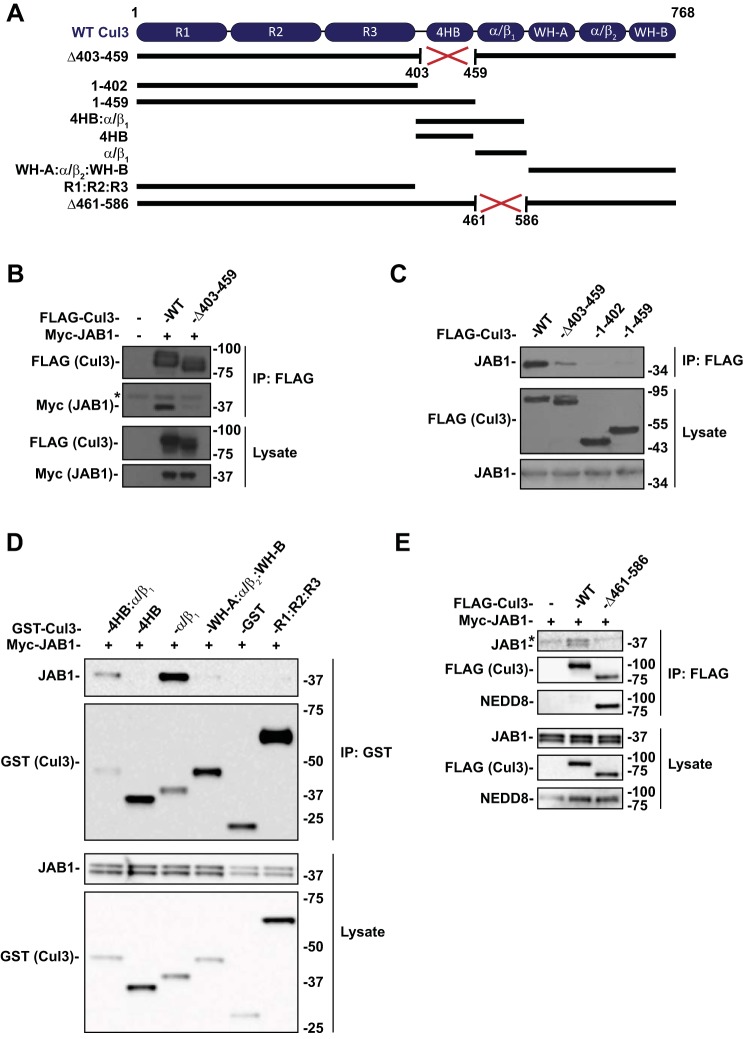
Fig. 1.
CSN binds to Cul3 at the α/β1 domain. A: Diagram of Cul3 domain structure and schematic of the Cul3 constructs. B: Coimmunoprecipitation was performed with HEK293 cells transfected with myc-JAB1 and FLAG-tagged WT Cul3 or Cul3Δ403–459 and analyzed by immunoblot. Cul3Δ403–459 exhibited a decreased interaction with JAB1 compared with WT Cul3. C: Effects of Cul3Δ403–459 on JAB1 binding was determined by coimmunoprecipitation of HEK293 cells with N-terminal domain Cul3 constructs using anti-FLAG and analyzed by immunoblot. Coimmunoprecipitation of N-terminal domain Cul3 constructs with (1–459) and without (1–402) the 4HB domain showed no binding to JAB1. D: Segments of the Cul3 protein were generated with a GST tag and cotransfected with myc-tagged JAB1 in HEK293 cells. Coimmunoprecipitation was performed using glutathione sepharose beads. Immunoblotting for JAB1 showed binding to 4HB:α/β1 and α/β1 Cul3 constructs but not to 4HB, WH-A:α/β:WH-B, or R1:R2:R3 Cul3 constructs. E: Coimmunoprecipitation was performed in HEK293 cells with myc-JAB1 and FLAG-tagged WT Cul3 or Cul3Δ461–586 constructs. Cul3Δ461–586 demonstrated less binding to JAB1 protein compared with WT Cul3. Immunoblotting for NEDD8 showed enhanced neddylation of the Cul3Δ461–586 construct compared with WT Cul3. *Nonspecific band. 4HB, 4-helix bundle; Cul3, cullin 3; CSN, COP9 signalosome; GST, glutathione S-transferase; HEK, human embryonic kidney; IP, immunoprecipitation; JAB1, jun activation domain-binding protein-1; NEDD8, neuronal precursor cell expressed developmentally downregulated protein 8; WT, wild type.