Abstract
Recent advances in understanding the regulation of amniotic fluid volume (AFV) include that AFV is determined primarily by the rate of intramembranous absorption (IMA) of amniotic fluid across the amnion and into fetal blood. In turn, IMA rate is dependent on the concentrations of yet-to-be identified stimulator(s) and inhibitor(s) that are present in amniotic fluid. To put these concepts in perspective, this review 1) discusses the evolution of discoveries that form the current basis for understanding the regulation of AFV, 2) reviews the contribution of IMA to this regulation, and 3) interprets experimentally induced shifts in AFV function curves and amnioinfusion function curves in terms of the activity of the amniotic fluid stimulator and inhibitor of IMA. In the early 1980s, it was not known whether AFV was regulated. However, by the late 1980s, IMA was discovered to be a “missing link” in understanding the regulation of AFV. Over the next 25 years the concept of IMA evolved from being a passive process to being an active, unidirectional transport of amniotic fluid water and solutes by vesicles within the amnion. In the 2010s, it was demonstrated that a renally derived stimulator and a fetal membrane-derived inhibitor are present in amniotic fluid that regulate IMA rate and hence are the primary determinants of AFV. Furthermore, AFV function curves and amnioinfusion function curves provide new insights into the relative efficacy of the stimulator and inhibitor of IMA.
Keywords: amniotic fluid, amniotic fluid volume, amniotic fluid volume function curves, intramembranous absorption, vesicular transport
INTRODUCTION
An amniotic fluid volume (AFV) in the normal range promotes the development of a healthy fetus. Presently, AFVs outside of the normal range occur in 5–10% of the ~4,000,000 births in the United States each year. These aberrations are associated with a wide variety of pregnancy complications and poor outcomes that lead to lifelong disease (36, 68, 75–77, 86, 93). Although approximately half of the fetuses with abnormal AFVs have comorbidities, the deleterious effects of nonnormal AFVs are independent of the presence of comorbidities and occur in fetuses that are normal except for the aberrant AFV (59, 80, 100). Because of the association between poor pregnancy outcome and abnormal AFV, it has long been accepted that understanding the mechanisms that regulate AFV is essential for the development of successful treatments to mitigate the deleterious effects on the fetus of oligohydramnios [too little amniotic fluid (AF)] and polyhydramnios (too much AF). Although there have been steady albeit slow increases in our understanding of the mechanisms that regulate AFV, there remains much to be understood, especially in primates, including humans. This review summarizes our perspective on the evolution of concepts of the mechanisms that regulate AFV and presents a new methodology for advancing understanding of the volume regulatory mechanisms. Descriptive studies that do not contribute to understanding of AFV regulatory mechanisms are not considered in detail. Instead, the chronology of evolving regulatory concepts is reviewed in hopes that the sequence of discoveries will stimulate further exploration.
EVOLUTION OF CONCEPTS
Early Concepts
In the early 1980s, it was not known whether AFV was in fact regulated. The reasons for this are complex: AF was difficult to access, especially over the long durations necessary to explore regulatory mechanisms; there were no widely used animal models for experimental studies of AFV regulation; and there were few studies of AF dynamics that provided mechanistic insights. Reviews before the 1980s (65, 88, 89, 101), although providing a wealth of information, were largely summaries of descriptive studies that did little to address the issue of volume regulatory mechanisms. The basic concept was that, rather than being a stagnant pool of fluid, AF turned over about once per day because of fluid inflows and outflows. Furthermore, many of the concepts were speculative rather than factually based. The relative contributions of the mother, the placenta, and the fetus to AFV regulation had received little attention and continue to require clarification and exploration.
One obstacle to understanding AFV regulation was that, as synthesized by Seeds in 1980, there are eight potential pathways through which water and/or solutes can move into and/or out of the AF (89). Furthermore, the relative contributions of these pathways to AFV change across gestation. For example, AF is present before the fetus begins to excrete urine into the amniotic sac, whereas fetal urine is the primary source of AF in late gestation. Mechanistically, to fully understand the regulation of AFV, volume flows and solute fluxes through each of these eight pathways need to be measured simultaneously under different experimental conditions, clearly an impossible task because of access, technical limitations, and inability to measure flows simultaneously.
Experimental Model for Exploring AFV Regulation
To address this complexity, we took advantage of two factors (97–99). First, the late-gestation fetal sheep experimental model allows chronic catheterization and AF sampling over many days. Second, after fetal skin keratinization and umbilical cord thickening during midgestation (removing the contribution of 2 pathways associated with transudation of water across the fetal skin and umbilical cord), there remain only six potential pathways in late gestation for water and/or solutes to move into and/or out of the amniotic space: fetal urine and lung liquid entry into the AF, fetal oral/buccal secretions into AF, fetal swallowing of AF, volume absorption plus solute exchange between AF and fetal blood perfusing the surface of the placenta (intramembranous transfer), and volume absorption plus solute exchange between AF and maternal blood within the wall of the uterus (transmembranous exchange).
Developmental Aspects of Inflow and Outflow Pathways
In the early 1980s, because of technical limitations at that time, only two of the six flows could be simultaneously determined. An important observation was that, similar to indirect estimates for human fetuses (1, 89), late-gestation fetal sheep produce a larger volume of urine each day than the volume of AF that is swallowed (99). At that time, there was no clear indication as to how that excess fluid left the amniotic sac. Although it was suggested that the fetal lungs absorbed AF (89), that clearly is not the case under normal conditions. The rare presence of meconium below the vocal cords in newborns despite the frequent occurrence of meconium in the AF together with direct measurements in fetal sheep show that the fetal lungs do not normally absorb fluid but rather secrete liquid that potentially contributes to AF (65, 78). Absorption of fluid by the fetal lungs occurs only under highly pathological conditions and is associated with elevated levels of stress hormones (including AVP) circulating in the fetus (38, 64). In the absence of pulmonary absorption, the only remaining possibilities that could explain the imbalance in urine production and swallowing are intramembranous absorption (IMA) into the fetal circulation and transmembranous absorption into the maternal circulation. This conclusion was further supported by the observation that daily spontaneous changes in AFV in sheep were only 47% correlated with daily urine volume and swallowed volume (99). Thus 53% of the daily AFV changes were unexplained and dependent on nonrenal, nonswallowing, nonpulmonary pathways. Another important observation in those early studies was that if fluid was added to or removed from the amniotic compartment, AFV gradually returned toward normal over a few days (99). The latter observations clearly suggest that AFV is regulated but provide no indication of the mechanisms.
Discovery of Intramembranous Absorption
In the late 1980s, warm distilled water was injected into ovine AF to explore the relative contribution of IMA versus transmembranous absorption to the regulation of AFV (51). Fetal blood osmolality decreased more rapidly and more extensively than maternal blood osmolality, demonstrating direct absorption of amniotic water into fetal blood. Furthermore, if the fetal esophagus was ligated, fetal blood osmolality fell even more rapidly than when the esophagus was intact (51). Thus swallowing of AF was not responsible for the rapid decrease in fetal osmolality after intra-amniotic water injection. Although it was speculated that absorption of AF directly into fetal blood may play a role in human AFV regulation (2), our study was the first to experimentally demonstrate that absorption of AF across the amnion and into fetal blood occurs rapidly, and we termed that process “intramembranous absorption.” Furthermore, if the fetus was killed immediately before the intra-amniotic water injection, maternal blood osmolality was unchanged over time, demonstrating that transmembranous absorption is volumetrically unimportant. The latter conclusion is consistent with statements by Seeds in his classic review (89) that in humans “. . . only a very small net transfer of water could occur by . . . [the transmembranous pathway] . . . since the vascularity of maternal tissue in proximity to the chorion laeve and amnion is sparse.” To estimate intramembranous volume flow in response to the intra-amniotic water injection (51), we assumed that only water was absorbed through the intramembranous pathway, consistent with Seeds’ earlier review indicating that water crosses biological membranes only by osmotic or hydrostatic forces (88), and calculated IMA to be ~200 ml/day. At that time, the concept of vesicular transport of fluid across the amnion had yet to be developed.
Only Four Major Amniotic Flows in Late Gestation
If transmembranous absorption is not volumetrically important, then only five potential pathways of fluid and solute movements need be considered in late gestation in order to understand the regulation of AFV. Of these, fetal oral/buccal secretions seemed the least likely to be volumetrically important. A study in the early 1990s found that the late-gestation ovine fetal oral/buccal cavities secreted ~8 ml·day−1·kg body wt−1 (15). Although it is unknown what fraction enters the AF or is swallowed before entering the AF, this volume flow is so small in comparison with other primary amniotic flows (described below) that it can be ignored. Furthermore, oral/buccal flow into AF appears to be approximately the same magnitude as estimated transmembranous outflow (6) but oppositely directed, so oral/buccal and transmembranous flows tend to cancel the effect of each other on AFV. This conclusion is important because it suggests that only two amniotic inflows and two outflows need be considered to understand the regulation of AFV in late gestation: fetal urine and lung liquid entry into the AF plus swallowing and intramembranous removal of AF.
Maternal, Placental, and Fetal Contributions to AFV Regulation
It is important to put in perspective the relative contributions of the mother, placenta, and fetus to the regulation of AFV. The issues are complex because of a multitude of conflicting observations and speculations. For example, from measurements of placental permeabilities of sodium and chloride in sheep, it was concluded that ovine placental permeabilities are so low that they are primary factors limiting fetal growth and hence AFV (39). That conclusion conflicted not only with early observations that large amounts of sodium and chloride crossed the ovine placenta from ewe to fetus in response to fetal urine drainage (56) but also with the observations that sodium and chloride rapidly crossed the placenta from fetus to ewe during a 3-day infusion of isotonic saline into the AF or fetal circulation (13, 50). To directly test whether sodium and chloride rapidly cross the placenta from fetus to ewe, we infused hypertonic NaCl into the fetal circulation at 2 ml/h (80% of fetal body sodium and chloride content per day) for 3 days (81). At the end of the 3-day experiment, although there were large increases in fetal urine production over time, combined amniotic and allantoic fluid volumes were normal and all the infused sodium and chloride had transferred to the maternal circulation. It must be concluded that ovine placental electrolyte permeabilities do not limit the ability of the fetus to transfer sodium and chloride across the placenta to the ewe and also that the fetus is able to maintain normal AFVs in the face of large increases in urine flow into the amniotic compartment. These observations suggest that the fetus is the primary regulator of AFV under a majority of circumstances.
In contrast, in response to fetal intravascular infusion of hypertonic sodium lactate for 3 days, plasma lactate concentration increased only modestly (from a basal level of 1.5 mmol/l to 6 mmol/l) while combined amniotic plus allantoic fluid volumes increased by an average of 4.5 liters (82), analogous to severe polyhydramnios. Fetal anemia sufficiently severe to elevate fetal plasma lactate concentration also elevates AFV (95). Unexpectedly, AFV was unchanged in response to intra-amniotic lactate infusion (87). We concluded that relatively small molecules such as lactate exert powerful osmotic effects on the placenta, drawing fluid from the maternal to the fetal compartment, whereas sodium and chloride do not. This osmotic mechanism appears to be responsible for the association between maternal diabetes and polyhydramnios in pregnant women (26). Furthermore, elevated AF lactate levels do not exert sufficient osmotic effects on the intramembranous pathway to alter AFV, presumably because of high intramembranous permeabilities (71). The increased AFV during elevated fetal plasma lactate is one of only two conditions that we have observed in which the placenta appears to be the major determinant of AFV in that the fluid osmotically attracted across the placenta from the mother to the fetus was efficiently dumped by the fetus into the AF.
The other condition in which the placenta may dominate the AFV response is during infusion of subpressor amounts of angiotensin into the fetal circulation (5, 41, 42). Extensive polyhydramnios develops in response to a 7-day angiotensin infusion in the absence of changes in fetal or maternal plasma osmolyte concentrations or osmolalities (7, 74). It appears that the accumulation of excess fluid in the fetal compartment is due to the effects of angiotensin on placental fluid transfer, since hydrops fetalis (gross fetal edema) occurs in response to a comparable angiotensin infusion in nephrectomized ovine fetuses (7, 40).
Somewhat surprisingly, the contribution of the mother to maintenance of AFV has received relatively little attention. In 1990, a study in pregnant sheep found that 54 h of water deprivation resulted in a 40% reduction in AFV in association with elevations in maternal and fetal blood osmolality (94). This is consistent with an earlier study showing that acute maternal hyperosmolality rapidly draws fluid from fetus to ewe (103). Furthermore, the near-term reversal of pregnancy-induced maternal hypoosmolality appears responsible for the predelivery decrease in AFV in mice and rats (29, 37). Beginning in 1990 (62, 92), several studies have shown that oral hydration with 1–2 liters of water increases the amniotic fluid index (an ultrasonic index of AFV) in humans with oligohydramnios or normal AFV. Although the increase in AFV was initially suggested to be independent of fetal urine production (46), a subsequent study has shown that an increase in fetal urine production is involved (79). The current consensus is that both maternal oral and intravenous hydration with hypotonic fluids increase AFV in humans with oligohydramnios as well as normohydramnios (55), but the underlying cause of the oligohydramnios has yet to be addressed. Whether there is a defect in placental water transfer or in maternal osmoregulation are questions that need exploration in order to understand the mechanisms by which the mother participates in AFV regulation/dysregulation.
Contribution of Lung Liquid Secretion to AFV
To begin to understand the contribution of each of the four major amniotic inflows and outflows to the regulation of AFV, one other major hurdle had to be overcome, that is, the potential contribution of lung liquid secretion to AFV. It was shown in 1974 (78) and in subsequent studies that the fetal lungs normally secrete large volumes of liquid equal to 6–10% of fetal body weight each day (58). Although it was accepted that lung liquid entered the AF because of the presence of lung surfactants in AF in late gestation, early studies in anesthetized ovine fetuses suggested that all the secreted lung liquid was swallowed as it exited the trachea (3). To address this apparent conflict, ovine fetal swallowed volumes and solute concentrations of swallowed fluid, lung liquid, and AF were measured five times daily over three consecutive days of normoxia, hypoxia, and normoxia. Based on daily swallowed volumes and solute concentrations, an average of 47% of the pulmonary secretions was swallowed as it exited the trachea, and the remainder entered the AF. Furthermore, 18% of the total swallowed volume was lung liquid under both normoxic and hypoxic conditions (17). Thus significant volumes of lung liquid enter the AF daily in late gestation under normal and nonnormal conditions and potentially contribute to AFV.
Early Estimates of IMA Rate
One of the disadvantages of the fetal sheep model for studying the regulation of AFV is that there are two extrafetal fluid compartments, the amniotic and the allantoic. Both of these compartments normally receive fetal urine (84, 102), confounding interpretation of AF dynamics in this model. To circumvent the problem, in the early 1990s we began ligating the urachus at the base of the umbilical cord in our fetal sheep preparations. With a ligated urachus, all fetal urine enters the amniotic sac and allantoic fluid volume decreases to zero or near zero within 2–3 days. With experimental studies starting 5 days after urachal ligation, the presence of the empty allantoic sac can be ignored for the purpose of data interpretation relative to AFV regulation.
To explore the IMA pathway with this experimental model with ligated urachus, we drained fetal urine to the exterior while simultaneously occluding the fetal trachea and esophagus, thereby eliminating all amniotic inflows and outflows except IMA (60). Over 8 h, AF solute concentrations and osmolality increased by ~10%. By assuming that only water (and not AF solutes) was absorbed intramembranously, IMA rate (IMAR) was (under)estimated to be 240 ml/day. In a subsequent study utilizing similar experimental procedures in which the change in AFV over 8 h was measured directly, AFV decreased by 128 ml or ~400 ml/day (18). In addition to the water, the major AF solutes (sodium, potassium, chloride, calcium, bicarbonate, glucose, and lactate) were being absorbed concomitantly through the intramembranous pathway. The amounts of solutes absorbed correlated positively with the volume absorbed and negatively with their respective concentration gradients between AF and fetal blood. In other words, there are two components of net intramembranous solute movement: a movement of solutes out of the AF with the volume of fluid transported intramembranously and a diffusional movement of solutes into AF from fetal blood that was dependent on concentration differences. Furthermore, the diffusional movement of solutes into AF was only a small fraction of the volume-dependent solute movement outward (18, 49). Unlike intramembranous solute transport that separates into passive (inward) and unidirectional (outward) fluxes, the relative contribution of passive intramembranous movement of water to total volume flux has been difficult to determine and needs further exploration. Passive water flow has been estimated to be 10–20% of total volume flow through the intramembranous pathway under basal conditions and a much smaller fraction when IMAR is elevated (18, 49).
Concept of Intramembranous Vesicular Transport
An important insight from the latter and other studies was that the net intramembranous movement of solutes out of the AF occurs against their respective concentration gradients. Another important factor is that AF pressure is lower than fetal vascular pressures, so IMA of amniotic water and solutes occurs against hydrostatic gradients (18, 49). Furthermore, although IMA normally occurs with the osmotic gradient from AF to fetal blood (AF has the lower osmolality), Faber and Anderson (43) found that elimination of the osmotic gradient between AF and fetal blood only slightly reduced IMAR. Based on these observations that IMA occurs against concentration gradients, against hydrostatic gradients, and in the absence of osmotic gradients, a major conclusion was that the underlying biological process is unidirectional vesicular transport of AF in bulk (18). This concept of bulk AF transport is consistent with amnion being a transport epithelium (90), subsequent studies of intramembranous solute absorption (49), and computer simulations of intramembranous volume and solute fluxes (23). Furthermore, observations that the amnion and amniochorion have similar permeabilities (66) and that permeability of the isolated amnion is similar to that of the intramembranous pathway (4) demonstrate that the amnion is the rate-limiting barrier of intramembranous transport.
Direct Determination of Normal Physiological IMA Rate
A large part of the challenge in understanding the regulation of AFV is the technical difficulty in experimentally determining IMAR. Studies before 2009 could only estimate IMAR either by eliminating some of the amniotic inflows and outflows (which alters normal IMAR) or by making assumptions about nonmeasured inflows and outflows. A methodological breakthrough occurred in 2009 with the development of an improved fetal sheep model that permitted three of the four major amniotic flows to be determined simultaneously over time (83). By measuring AFV at the beginning and end of the experimental periods (typically 2–3 days), average IMAR over the experimental period could be calculated with the mass balance equation (83):
| (1) |
where ∆AFV is the change in AFV over time, UrVol is the volume of fetal urine that entered the AF, LuVol is the volume of lung liquid that entered the AF, and SwVol is the volume of AF swallowed by the fetus over the same time period. For experiments in which urine or lung liquid was continuously drained to the exterior and isovolumically replaced with lactated Ringer solution, UrVol and LuVol are the volumes of Ringer solution infused to replace the endogenous fluids. With four of the five variables in Eq. 1 measured experimentally, IMAR can be calculated for the time period over which measurements were made and averages 700–1,000 ml/day in late-gestation ovine fetuses (Discovery of Stimulatory and Inhibitory Factors for Intramembranous Absorption). Such simultaneous measurements of four experimental variables and determinations of IMAR have been made only in late-gestation ovine fetuses.
Primary Regulator of AFV
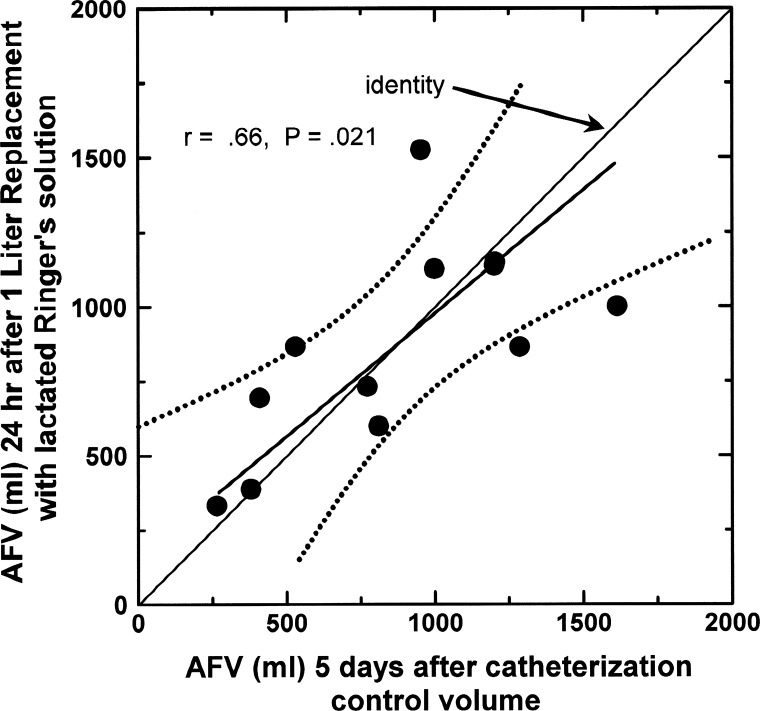
With this improved model, it is possible to demonstrate that, even though each amniotic inflow or outflow can contribute to changes in AFV over time, IMAR is the primary determinant of AFV (9, 23, 35, 42, 44, 96). This is illustrated in Table 1 by the changes in AFV and the four amniotic flows in response to amnioinfusion of lactated Ringer solution at a rate of 1.39 ml/min (2 l/day) for 2 days. At the end of the infusion, AFV increased by only 38.8% of the infused volume, so it is clear that AFV was being regulated to maintain volume near normal. However, rather than decreasing to compensate for the infusion, fetal urine production and lung liquid secretion increased, resulting in a total fluid challenge into the amniotic compartment of 134% of the infused volume. Consistent with previous studies (22, 25), fetal swallowing increased during the infusion, accommodating nearly 21% of the infused volume. By far the largest compensatory response was that IMA increased by 74% of the infused volume. This, combined with prior observations (35, 44, 83, 96), represents the basis for concluding that AFV is regulated predominantly by variations in IMAR. In this review, we also utilize the observation that AFV stabilizes in 1–2 days after a volume disturbance (23) to interpret relationships between variables under presumed steady-state conditions. This is illustrated by the correspondence between AFV before and 24 h after AF drainage and replacement with 1 liter of lactated Ringer solution (Fig. 1). As shown, AFV returned to a volume close to that before replacement irrespective of whether the volume was below or above 1 liter initially.
Table 1.
Fluid responses to intra-amniotic infusion of lactated Ringer solution in late-gestation sheep
| Fluid Change | Volume, ml (mean ± SE) | Percentage | Total |
|---|---|---|---|
| Infused volume | 4,191 ± 195 | 100% | |
| ΔUrine flow | 1,123 ± 302 | +26.8 | |
| ΔLung fluid secretion | 300 ± 48 | +7.2 | |
| 134% | |||
| ΔAmniotic fluid volume | 1,628 ± 294 | +38.8 | |
| ΔSwallowed volume | 867 ± 236 | +20.7 | |
| ΔIntramembranous absorption | 3,120 ± 477 | +74.4 | |
| 134% |
Fluid responses to intra-amniotic infusion of 2 l/day of lactated Ringer solution for 2 days in 6 late-gestation sheep are shown (data from Ref. 24). Percentages are expressed relative to infused volume.
Fig. 1.
Amniotic fluid volume (AFV) before and 24 h after draining of amniotic fluid and replacement with 1 liter of lactated Ringer solution in late-gestation ovine fetuses with ligated urachus. Data from Refs. 9 and 24. Each circle represents a different fetus. The slope of the regression line is not different from unity, and the pre- and postreplacement volumes are not different by paired t-test. Dotted lines show the 95% confidence interval.
Intramembranous Solute Fluxes
With the improved fetal sheep model came new insights into intramembranous solute transport. By comparing individual intramembranous solute flux rates against intramembranous volume flux rates, not only were passive intramembranous permeabilities of solutes determined but we also found that the slope of the regression equation for each solute equaled the AF concentration of that solute (49). In other words, the fluid being transported presumably by vesicles across the amnion had the same concentrations of solutes as AF, confirming our earlier speculation that transamnion vesicular transport was bulk transport of AF (18). A new observation was that the intercept of the solute flux vs. volume flux regression equation was unchanged under different experimental conditions (49). The interpretation is that passive permeability characteristics of the intramembranous pathway of solutes such as sodium and chloride are unchanged under experimental conditions when there are simultaneous large changes in vesicular transport rate (35, 49).
The lack of passive solute permeability changes of the intramembranous pathway under experimental conditions also appears to be true for passive water movement in that the gene expression of five aquaporins in the amnion was unchanged during both increases and decreases in IMA (32). Although unchanged with experimental conditions, amnion aquaporin 1 protein levels correlated with IMAR, suggesting that aquaporin 1 may play a role in regulating passive water transport through the amnion under basal conditions. This is consistent with studies showing that aquaporin 1 knockout is associated with altered AFV in mice (67, 72, 104). Interestingly, in fetal sheep tubulin-α mRNA levels in the amnion correlated positively with IMAR (33), suggesting that, rather than altering the number of transport vesicles, changes in IMAR are produced at least in part by altering the cytoskeletal structure along which vesicles are transported. Unexpectedly, amnion caveolin-1 mRNA and protein levels are reduced under hypoxic conditions when fetal urine production and IMAR are greatly increased (30, 96), suggesting that caveolae are not the major transport vesicle of IMA.
Induced Molecular Changes Within Amnion
In the absence of indications as to the identity of the regulators of IMAR, a series of studies was conducted to identify changes within the ovine amnion that occur when IMAR is altered. Four days of moderate fetal hypoxia produced large increases in fetal urine production and IMAR, little change in AFV or swallowing, and simultaneous increases in amnion VEGF mRNA levels (73, 96). Similarly, intra-amniotic infusion of physiological saline for 3 days produced only small increases in AFV compared with the volume of infused fluid and simultaneously elevated amnion VEGF mRNA levels (35). This led to our speculation that VEGF may play a primary role in regulating IMAR (28). However, recent studies found that amnion VEGF mRNA levels are also elevated when IMAR is experimentally reduced (33), suggesting that VEGF may not be a major regulator of IMAR.
Contribution of Fetal Swallowing, Fetal Urine Excretion, and Lung Liquid Secretion to AFV
Although the regulation of fetal urine production, swallowing, and lung liquid secretion has been widely explored, their contributions to the regulation of AFV had received little attention except under extreme conditions in which fetal urine drainage without fluid replacement leads to oligohydramnios (63) and esophageal ligation results in polyhydramnios (47, 61). Because physiological regulation of fetal urine production, swallowing, and lung liquid secretion occurs in order to meet the needs of fetal body fluid balance rather than to maintain AFV, a 1999 National Institute of Child Health and Human Development-sponsored workshop on AF biology (85) concluded that if AFV is regulated the regulation would occur through modulation of IMAR, even though IMAR was not known to be regulated at the time.
To address the potential physiological roles of fetal swallowing, urine excretion, and lung liquid secretion in AFV regulation, we conducted a series of studies. With continuous drainage of lung liquid to the exterior and isovolumic replacement with lactated Ringer solution, AFV was unchanged over time both during basal conditions and during fluid volume loading into the AF (83), indicating that fetal lung liquid contains neither a stimulator nor an inhibitor of IMA and thus does not play a direct role in regulating IMAR and hence AFV. The effects on AFV of lung liquid drainage without fluid replacement have yet to be explored but would provide insight into the passive contribution of lung liquid to AFV.
In contrast to minimal changes in lung liquid secretion under many experimental conditions, fetal swallowing was reduced when AFV was low and increased to a maximum of twice normal as AFV was elevated (22, 25), resulting in a reduced rate of AF removal when AFV is low and an increased AF removal when AFV is high. Thus fetal swallowing plays an important regulatory role in maintaining AFV near normal as AFV changes. However, the mechanisms by which fetal swallowing varies with AFV remain to be determined, although we speculated that the fetus may be more able to open its mouth and swallow as AFV increases and vice versa (22, 25).
Fetal urine contributes to AFV in two ways: by delivering 1) a volume of fluid and 2) chemicals to the amniotic compartment (described below). Fetal urine production correlates positively with AFV, being low when AFV is low and elevated when AFV is high (20). Under normal conditions, urine entry into the AF is essential for AFV maintenance because in the absence of urine entry into the AF anhydramnios typically results, especially in late gestation. Furthermore, intra-amniotic infusions of physiological saline elevate urine production when a reduction in urine production is needed to help maintain normal AFV (83). Thus the volume of urine excreted by the fetus is often opposite to that needed for maintenance of AFV near normal.
Vesicular Uptake in Human Amnion
In light of experimental observations, unidirectional vesicular transport of AF across the amnion was concluded to be the primary mechanism of IMA. In a recent study of uptake in cultured human amnion cells (91), specific molecular markers of receptor-mediated and non-receptor-mediated vesicular uptake displayed first-order saturation kinetics, with half-saturation times for the different markers averaging 0.5–2 min at 37°C. Thus vesicular uptake in human amnion cells is sufficiently rapid to mediate IMA. Furthermore, endocytosis of specific markers suggested that caveolae and clathrin-coated vesicles are responsible for fluid uptake. Further study is needed to better characterize the kinetics of endocytosis, transcytosis, and exocytosis of the amnion as well as vesicle types under varying experimental conditions.
Discovery of Stimulatory and Inhibitory Factors for Intramembranous Absorption
Even though basal IMARs of 700–1,000 ml/day were unexpectedly high in the improved fetal sheep model when IMAR was directly determined (9, 22, 24, 31–33, 83), infusion of lactated Ringer solution into the AF greatly increased IMAR (24, 83), consistent with several earlier studies showing increased IMAR in response to either intra-amniotic fluid infusions or fetal polyuria induced by intravascular fluid infusions (13, 35, 42–44). We had long suspected that the increased IMARs during fetal infusions were due to either dilution of a substance in the AF that was inhibiting IMA absorption or entry into the AF of a stimulator of IMA in response to the infusions. However, our early studies failed to support these possibilities (8, 19), for methodological reasons unknown at the time. In one study, AFV was expanded by AF infusion into the amniotic compartment (19). The infused AF had been frozen, stored, thawed, and rewarmed before reinfusion. In retrospect, it is likely that these treatments altered the stimulator and inhibitor of IMA that were in the infused AF, especially the stimulator, as it appears to have a short half-life in AF.
More recent studies show that AF contains a stimulator and an inhibitor of IMA. If fetal urine is continuously drained to the exterior and isovolumically replaced with lactated Ringer solution, IMAR drops to zero and AFV increases over time (9). That finding was a breakthrough in our understanding of the AFV regulatory mechanisms in that it showed that fetal urine contains a powerful stimulator of IMAR and that without the stimulator the vesicular component of IMA stops. The observation that fetal urine drainage without fluid volume replacement also results in an IMAR not different from zero (33) supports these concepts. The identity of the fetal urinary IMA stimulator has yet to be determined, but it is not prostaglandin E2 (31), the most prevalent prostaglandin in fetal urine. Furthermore, it is unknown whether renal excretion of the stimulator changes with condition when AFV is either spontaneously or experimentally altered.
The observation that fetal urine contains a stimulator of IMA is also important because it provides insight as to why estimated IMAR was much lower in some of our early studies (18, 60) compared with directly measured IMARs determined with the improved fetal sheep model. Those early studies included urine drainage to the exterior resulting in loss of the stimulator from the amniotic compartment. The differences in IMARs between those studies suggest that the stimulator has a short half-life in AF in that it appears to be rapidly cleared.
In an unexpected way, the fetal membranes contribute to the physiological regulation of AFV by secreting an inhibitor(s) of IMA into the AF. During lactated Ringer solution infusion into ovine AF, IMAR increased independent of whether fetal urine entered the amniotic sac or was continuously replaced with lactated Ringer solution (24). Because stretch of the ovine fetal membranes does not alter AFV (45), and because the response to infusion was not IMA stimulator dependent, the interpretation is that AF contains an inhibitor of IMA that was diluted by the intra-amniotic infusion. Because neither the lungs nor the fetal kidneys are the source of the inhibitor, it most likely is derived from the fetal membranes, although secretions from the fetal oral/nasal cavities may contribute. The presence of an IMA inhibitor in AF provides a basis for understanding IMAR increases in response to fetal polyuria or intra-amniotic fluid infusion in that the inhibitor would be diluted by the increased amniotic inflows. Furthermore, because IMAR after the intra-amniotic fluid infusion was independent of urine entry into the AF, it appears that the stimulator has a limited range of effectiveness.
AFV Regulation: Volume Regulated but Not Sensed
From the above, it is clear that AFV is being regulated even though AFV itself is not being sensed. Rather, AFV regulation occurs through changes in fetal swallowing of AF, urine production, and IMA. The latter occurs by altering the concentrations of the stimulator and inhibitor of IMA that are present in AF, somewhat analogous to the regulation of urine production by the kidneys. By combining into a computer model five of the above concepts [that 1) there are 4 major amniotic flows in late gestation, 2) IMA includes both passive and active components, 3) fetal urine delivers an IMA stimulator to the AF, 4) the fetal membranes secrete an inhibitor of IMA into the AF, and 5) IMAR is regulated by the concentration of the stimulator and inhibitor present in AF], we simulated the AFV and AF compositional responses to a wide variety of, but not all, experimental conditions (23). The next major hurdle is to identify the IMA stimulator and inhibitor. Analyses of amnion and AF molecules by proteomic and genomic approaches could lend insights into the identity of the responsible mediators and pathways that they regulate.
AMNIOTIC FLUID VOLUME FUNCTION CURVES AND AMNIOINFUSION FUNCTION CURVES
Function curves are used to demonstrate the dependence of a physiological variable on multiple, complex, and sometimes unknown regulatory factors and interactions. Examples include the determination of cardiac output by the intersection of cardiac function and venous return curves and the determination of left thoracic duct lymph flow rate as a function of central venous pressure during different states of hydration (11, 12, 14, 16, 57). Advantages of function curves include not only that they simplify presentation of the interdependence between variables but also that they may provide new insight into how a variable is regulated.
In this review, we define an AFV function curve as the dependence of AFV on IMAR under one of two conditions: either in the absence of exogenous fluid infusion into the amniotic sac or in the presence of exogenous fluid infusion at the same rate at which an endogenous fluid (urine or lung liquid) is being continuously drained to the exterior (isovolumic replacement). An amnioinfusion function curve is defined as the relationship between AFV and IMAR before and after intra-amniotic infusion of exogenous fluid. Furthermore, an AFV or amnioinfusion function curve can be derived for each experimental condition. Although a previous report on AFV regulation used the phrase “function curve” (44), IMAR was plotted as a function of AFV in that study. As our understanding of the regulation of AFV evolved, it became clear that AFV is a function of IMAR rather than vice versa.
Normal AFV Function Curve
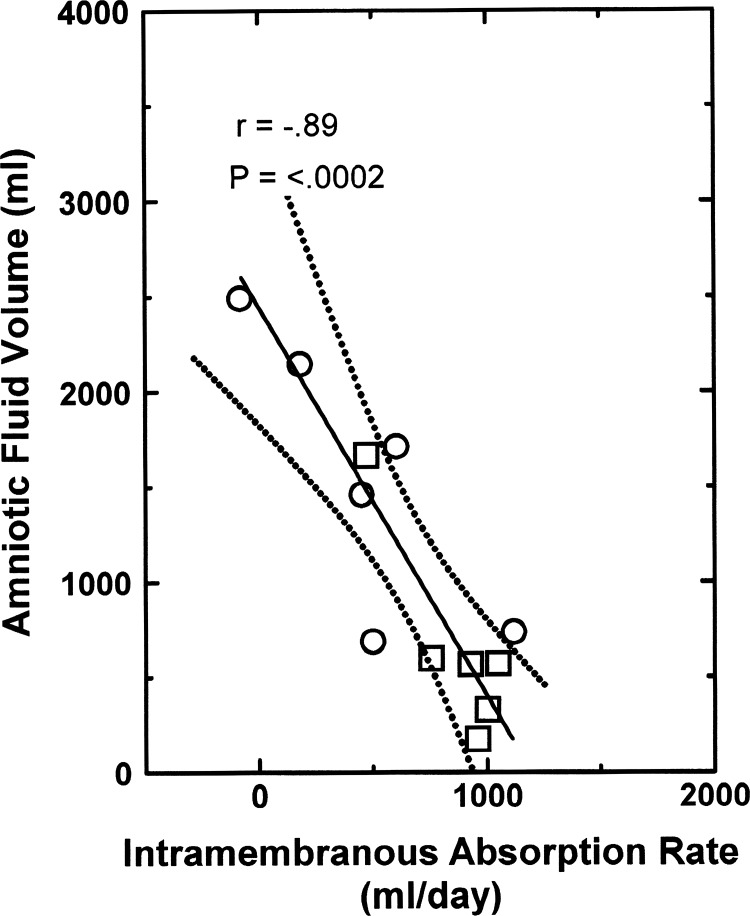
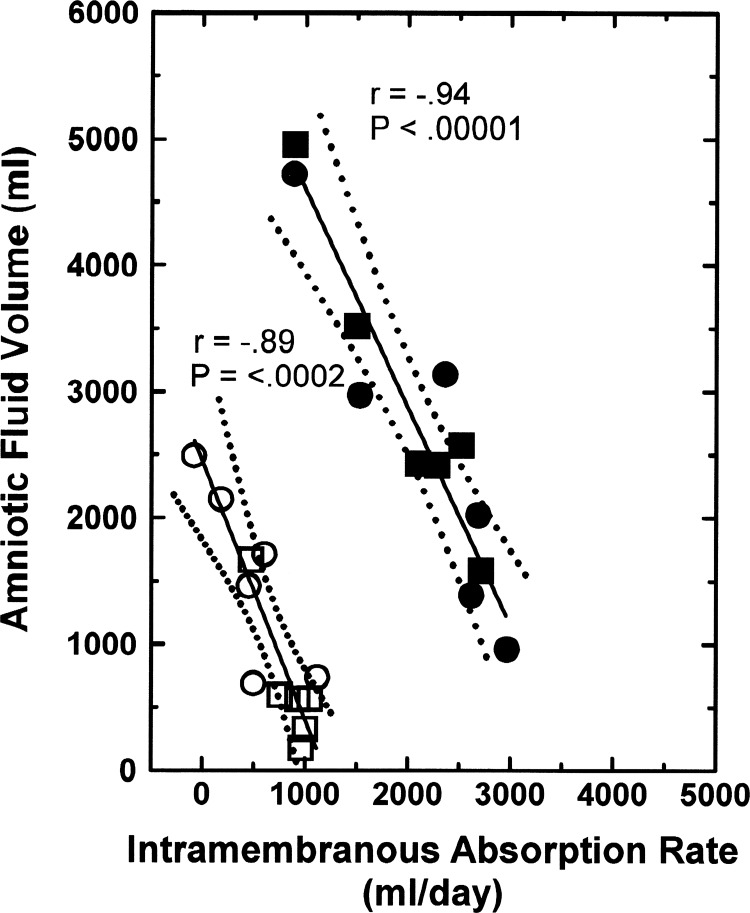
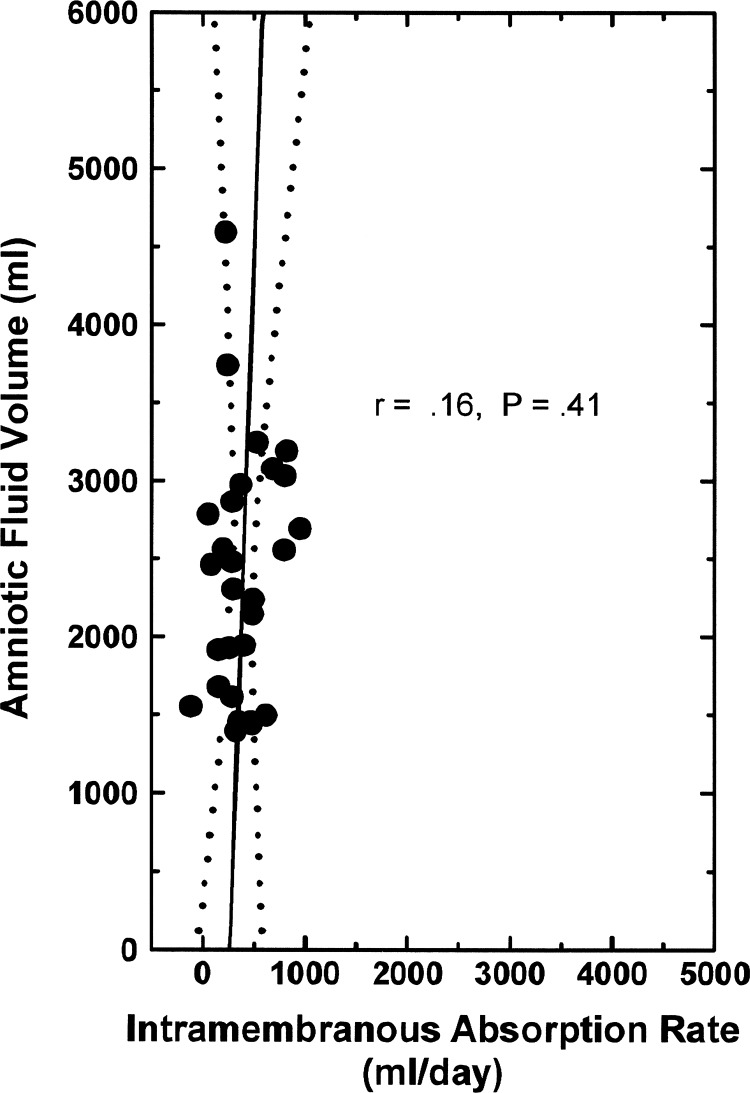
From the perspective that IMA removes AF, there is a negative relationship between the two variables under normal conditions (8, 23), as illustrated by the AFV function curve in Fig. 2. Furthermore, when fetal urine was drained to the exterior and isovolumically replaced with physiological saline (9), even though AFV increased and IMAR decreased the AFV function curve was unchanged [analysis of covariance (ANCOVA) P = 0.38, comparing the 2 groups]. The regression equation for the combined groups is
Fig. 2.
Normal amniotic fluid volume function curve: amniotic fluid volume as a function of intramembranous absorption rate in 6 ovine fetuses under control conditions (☐) and in the same fetuses when their urine was isovolumically replaced with lactated Ringer solution (○). Data from Ref. 9. Solid line is the regression line, and dotted lines show the 95% confidence interval.
Thus for every 1 ml/day decrease in IMAR, AFV increased by 1.8 ml and vice versa under steady-state conditions. Furthermore, the slope of the AFV function curve (1.81) indicates that IMAR is a powerful determinant of AFV.
Amnioinfusion Function Curve
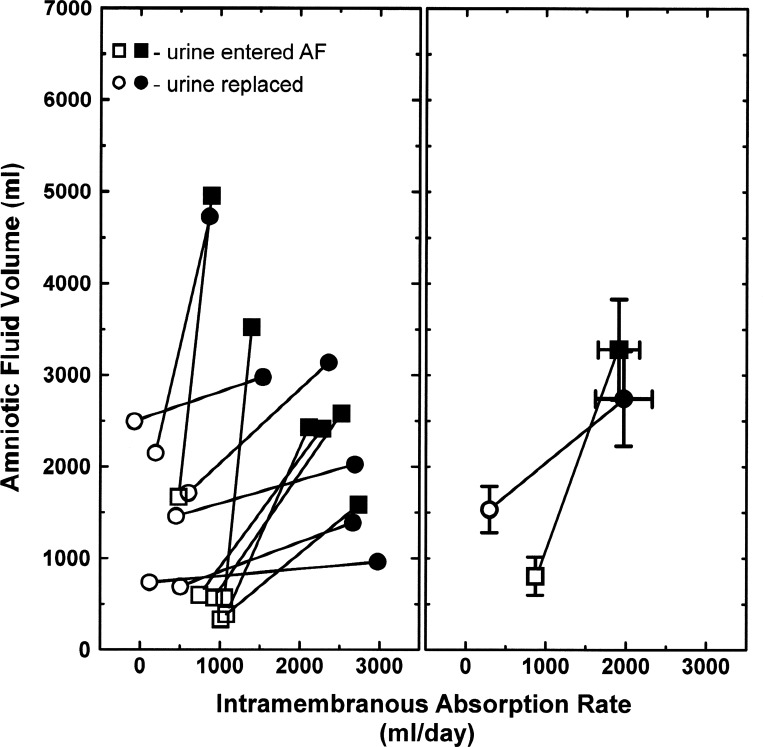
In response to amnioinfusion of 2 l/day of lactated Ringer solution for 2 days, AFV and IMAR increased in every fetus with fetal urine entering the AF or with fetal urine replacement (Fig. 3, left). These are referred to as amnioinfusion function curves, and the averages of these curves relating AFV and IMAR are shown in Fig. 3, right. From the latter averages, the amnioinfusion function curves are represented by the equations
in control fetuses and
in urine-replaced fetuses.
Fig. 3.
Amnioinfusion function curves. Amniotic fluid volume and intramembranous absorption rate before (open symbols) and after (filled symbols) intra-amniotic infusion of 4 l/2 days of lactated Ringer solution in individual ovine fetuses (left) and their average (±SE) responses (right). Fetal urine either entered the amniotic fluid (AF) or was isovolumically replaced with lactated Ringer solution. Data from Refs. 9 and 24.
It should be noted that although AFV and IMAR were different between the two groups before infusion (Fig. 3), AFV and IMAR at the end of the volume infusion were independent of whether fetal urine entered the amniotic compartment or was isovolumically replaced with lactated Ringer solution (Fig. 3, right). With similar IMARs at the end of the infusion in the normal and urine-replaced groups (Fig. 3, right), it appears that the urine-derived IMA stimulator present in AF was diluted by the volume infusion to the point that it was ineffective. Taken together, these results indicate that, in response to amnioinfusion, the increase in AFV was greater in control fetuses than in urine-replaced fetuses (2,226 ± 308 ml vs. 994 ± 357 ml, t-test P = 0.024) whereas the increase in IMAR was less (1,099 ± 245 ml/day vs. 1,865 ± 302 ml/day, t-test P = 0.033). This presumably is due to dilution of the IMA inhibitor in response to the elevated preinfusion AFV in the urine-replaced fetuses.
Volume-Loaded AFV Function Curve
In ovine fetuses after receiving an amnioinfusion of 2 l/day for 2 days, the AFV function curve shifted upward and to the right, and the negative relationship between AFV and IMAR remained (Fig. 4, top right). Furthermore, in volume-loaded fetuses, comparison between fetuses with urine entering the AF and those with isovolumic urine replacement showed that the two groups of fetuses are on the same AFV function curve (ANCOVA P = 0.73). The regression relationship for the combined two groups is
Fig. 4.
Normal and volume-loaded amniotic fluid volume function curves. Top right: volume-loaded function curve after infusion of 4 l/2 days (1.39 ml/min) of lactated Ringer solution in 6 fetuses in which urine either entered the amniotic fluid (■) or was isovolumically replaced with lactated Ringer solution (●). Bottom left: amniotic fluid volume function curve before amnioinfusion from Fig. 2 in 6 ovine fetuses under control conditions (☐) and in the same fetuses when their urine was isovolumically replaced with lactated Ringer solution (○). Data from Refs. 9 and 24. Regression (solid lines) and 95% confidence intervals (dotted lines) are shown.
When comparing this regression equation for the AFV function curve after intra-amniotic volume infusion with that in fetuses not receiving volume loading (Fig. 4, bottom left), the intercept for the infusion groups was shifted upward (ANCOVA P < 0.0001) by a volume equal to the infused volume (4,000 ml), while the slope was unchanged. Thus the normal and volume-loaded AFV function curves are parallel, suggesting that the intramembranous transport mechanisms are functioning similarly under the two conditions, except at a higher rate after volume loading. This is consistent with studies showing that for changes in IMAR both above and below normal, only the vesicular transport component of IMA changed, while passive intramembranous transport changed minimally if at all (9, 18, 21, 23, 49). The increased IMAR after volume loading corroborates the concept that amnioinfusion diluted the AF concentrations of both the intramembranous stimulator and the intramembranous inhibitor. However, if the inhibitor was dominant, then IMAR would increase as observed.
On the basis of the observed shift in the intercept and unchanged slope of the function curve in volume-loaded fetuses, AFV function curves after different amounts of volume loading can be predicted with reasonable confidence. For example, after a 1 l/day volume infusion for 2 days, the AFV function curve would be
Although function curves with different infusion volumes and durations require further exploration, an intra-amniotic infusion of 4 l/day of lactated Ringer solution for 3 days (83) resulted in a further shift upward and to the right in the AFV function curve, which remained parallel to the control AFV function curve.
AFV Function Curves During Blockade of Swallowing
A tracheo-esophageal shunt that prevented swallowing of AF and eliminated entry of lung liquid into the AF was placed in late-gestation ovine fetuses. Over the following 9 days, IMAR remained low as AFV increased because of the continued entry of urine into the amniotic compartment (61). The result was a very steep AFV function curve with AFV largely independent of IMAR because IMA increased little as AFV increased (Fig. 5). Under this condition, both the intramembranous stimulator excreted in fetal urine and the inhibitor secreted presumably by the fetal membranes would continue to enter the AF. The implication is that without removal of the inhibitor of IMA in AF by fetal swallowing, the effects of the inhibitor dominated and the fetal urinary stimulator was less effective. In other words, the intramembranous inhibitor appears to be more powerful than the stimulator. Although unexplored, as noted above, this could be due to rapid degradation of the stimulator in AF but may also be due to reduced excretion from the fetal kidneys or removal by the basal IMA that remained.
Fig. 5.
Amniotic fluid volume function curve in 7 ovine fetuses with a tracheo-esophageal shunt. Intramembranous absorption rate was calculated at 2-day intervals over a period of 9 days. Data from Ref. 61. Regression (solid line) and 95% confidence interval (dotted lines) are shown.
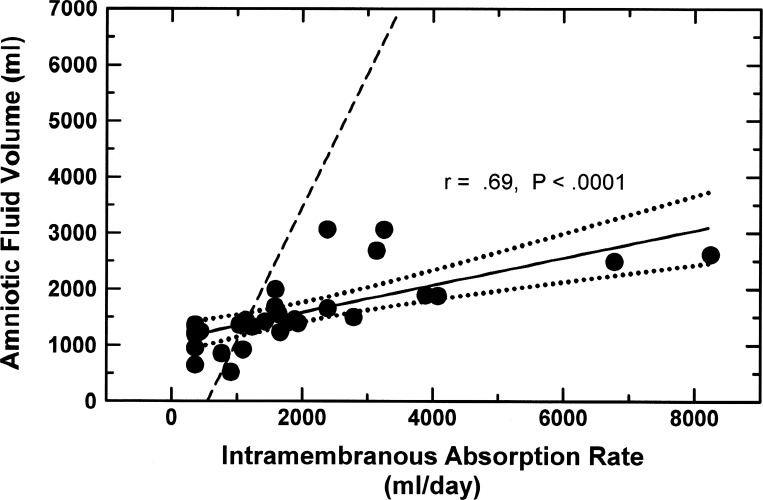
This function curve analysis also provides insights into our past study suggesting that AFV was not regulated by constituents within the AF (8). In that study, experiments were conducted in fetuses with tracheo-esophageal shunts. The above analysis (Fig. 5) shows that AFV regulation in the presence of a tracheo-esophageal shunt is very different from normal (Fig. 2). In that study AF was washed out by continuous infusion of 3 l/day of lactated Ringer solution for 3 days and withdrawal of AF at the same rate. AFV before the washout was only 62% of that after the washout (their Fig. 2). Thus AFV after washout was 161% (1/0.62) of the volume before washout. This demonstrates that AFV was significantly affected by the AF washout even though the AFV regulatory mechanisms were not functioning normally because of the presence of the shunt.
AFV Function Curves During Prolonged Fetal Hypoxia
During 4 days of sustained hypoxia in which fetal blood oxygen saturation was reduced by 40% (96), fetal urine production increased progressively to very high levels, averaging 5,840 ± 1,368 ml of urine entering the AF above basal levels over the 4-day period. Thus the effect of sustained hypoxia on AFV is somewhat comparable to prolonged amnioinfusion. Concomitantly, AFV increased by 885 ± 438 ml [analysis of variance (ANOVA) P = 0.0042] or 15% of the excess volume of urine after 4 days of hypoxia, while fetal swallowed volume was unchanged (96). Figure 6 compares the measured AFVs and estimated IMARs in hypoxic fetuses with the amnioinfusion function curve for normal fetuses. The regression relationship between AFV and IMAR is
for hypoxic fetuses. The slope of 0.234 during hypoxia contrasts with that of 2.38 for the amnioinfusion function curve in normal fetuses. Thus increases in IMA were 10 times (2.38/0.234) as effective at preventing increases in AFV during hypoxia as during intra-amniotic fluid infusion in normoxic fetuses. The reasons for this are unclear. The implications are that, in addition to the polyuria-induced dilution of the intramembranous inhibitor, hypoxia may have induced increases in secretion of the intramembranous stimulator and reduced secretion of the intramembranous inhibitor. Other changes in the membranes mediated by hypoxia are also likely, including changes in vesicular transport capacity by the amnion, all of which need exploration.
Fig. 6.
Amniotic fluid volume function curve in 6 ovine fetuses subjected to 4 days of hypoxic hypoxia (●). Data are daily values for 1 day before and 4 days after initiation of hypoxia in fetuses that did not become acidotic during the hypoxia (96). Intramembranous absorption rate estimated from Eq. 1 with measured amniotic fluid volumes, swallowed volumes, and urine volumes and estimating lung liquid secretion constant at 360 ml/day. Solid line is the regression line, and dotted lines are the 95% confidence intervals. Dashed line is amnioinfusion function curve of normal fetuses from Fig. 3.
Synthesis of AFV and Amnioinfusion Function Curves
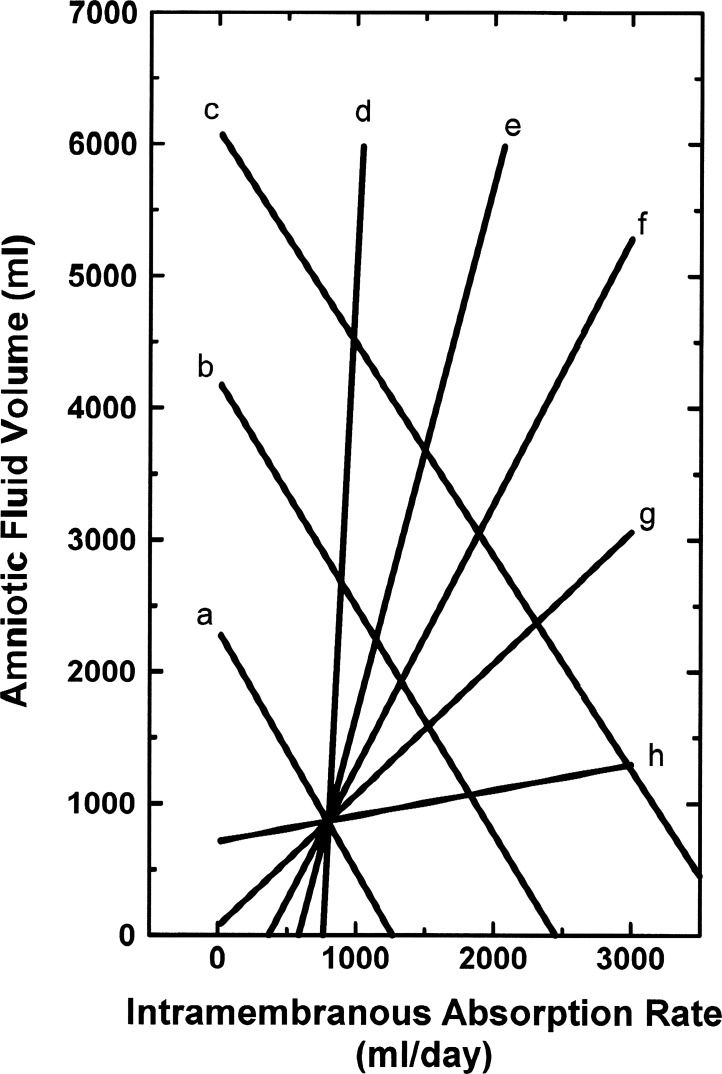
Figure 7 shows several hypothetical AFV and amnioinfusion function curves synthesized from the above analyses and illustrates the concept that AFV can be defined as the intersection of the AFV function curve and the amnioinfusion function curve. For example, the intersection of lines a and f in Fig. 7 approximates normal conditions in the late-gestation ovine fetus, where AFV = 872 ml and IMAR = 786 ml/day. The intersection of lines b and f in Fig. 7 represents conditions after a 4 l/2 days infusion, where AFV = 1,938 ml and IMAR = 1,319 ml/day. In normal fetuses, if IMAR were to decrease by a factor of 2, then AFV would approximately double to 1,584 ml from line a in Fig. 7, a condition analogous to polyhydramnios. If IMAR were to double on the normal AFV function curve (line a, Fig. 7), a condition would occur outside the possible ranges of AFV, as AFV cannot be less than zero. Instead, an AFV of zero would result, comparable to the condition of anhydramnios. If IMAR changed negligibly as amniotic flows into and out of the amniotic compartment varied (line d, Fig. 7), large fluctuations in AFV would result (a condition similar to blocked fetal swallowing; Fig. 5). Alternatively, under opposite conditions (line h, Fig. 7), little change in AFV would occur over a wide range of amniotic flows into and out of the amniotic compartment, as seen during conditions analogous to those induced by prolonged fetal hypoxia (Fig. 6).
Fig. 7.
Synthesis of amniotic fluid volume (AFV) function curves (lines a–c) and amnioinfusion function curves (lines d–h) representing 2-day experimental periods in ovine fetuses with urine entering the amniotic fluid derived from Figs. 3, 4, 5, and 6. Line a is the normal AFV function curve, line f the normal amnioinfusion function curve, line b the AFV function curve after a 2 l/2 days fluid volume load, and line c the AFV function curve after a 4 l/2 days volume load; lines d, e, f, g, and h are amnioinfusion function curves with slopes 10, 2, 1, 0.5, and 0.1 times normal.
Perspectives and Significance
Abnormal AFVs occur in 200,000–400,000 pregnancies in the United States each year and can directly cause a variety of clinical problems as well poor pregnancy outcomes. From studies in the fetal sheep model, it is clear that AFV is determined primarily by the IMAR under normal and abnormal conditions, including clinically relevant disorders such as placental insufficiency and fetal hypoxia (48, 96). Rapid IMA also occurs in fetal monkeys (54) and likely is an important determinant of AFV in humans (2, 10, 34, 69). In turn, IMAR is regulated by the concentrations of the stimulator and inhibitor of vesicular transport that are present in the AF. The next major advance would constitute understanding the characteristics of the stimulator(s) and inhibitor(s) of IMA as well as the molecular pathways they regulate. Once identified, it may be possible to physiologically and/or pharmacologically intervene clinically to correct aberrant AFVs to any desired volume by intra-amniotic administration of the stimulator or inhibitor. Alternatively, pharmacological agents can be administered intra-amniotically to alter endogenous levels of the stimulator and/or inhibitor. This approach is possible because drugs and hormones appear rapidly in fetal blood after intra-amniotic injection and are biologically active (27, 52, 53, 70). Conditions of polyhydramnios could be reversed by either reducing the concentration or production of the inhibitor or increasing the concentration or production of the stimulator in order to return AFV toward normal. The opposite could be applied if oligohydramnios is present. This potential therapeutic benefit provides compelling justification for the identification of the regulators of IMAR.
GRANTS
This work was supported in part by National Institute of Child Health and Human Development Grant 5R01 HD-061541.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.B. conceived and designed research; R.A.B., C.Y.C., and D.F.A. analyzed data; R.A.B., C.Y.C., and D.F.A. interpreted results of experiments; R.A.B. and D.F.A. prepared figures; R.A.B., C.Y.C., and D.F.A. drafted manuscript; R.A.B., C.Y.C., and D.F.A. edited and revised manuscript; R.A.B., C.Y.C., and D.F.A. approved final version of manuscript.
REFERENCES
- 1.Abramovich DR, Garden A, Jandial L, Page KR. Fetal swallowing and voiding in relation to hydramnios. Obstet Gynecol 54: 15–20, 1979. doi: 10.1097/00006250-197907000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Abramovich DR, Page KR. Fetal control of amniotic fluid volume in the human. In: Fetal Physiology and Pathology, edited by Belfort P, Pinotti JA, Eskes TK. Carnforth, UK: Parthenon, 1989, p. 9–14. [Google Scholar]
- 3.Adams FH, Desilets DT, Towers B. Control of flow of fetal lung fluid at the laryngeal outlet. Respir Physiol 2: 302–309, 1967. doi: 10.1016/0034-5687(67)90035-7. [DOI] [PubMed] [Google Scholar]
- 4.Adams EA, Choi HM, Cheung CY, Brace RA. Comparison of amniotic and intramembranous unidirectional permeabilities in late-gestation sheep. Am J Obstet Gynecol 193: 247–255, 2005. doi: 10.1016/j.ajog.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DF, Faber JJ. Animal model for polyhydramnios. Am J Obstet Gynecol 160: 389–390, 1989. doi: 10.1016/0002-9378(89)90454-7. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DF, Borst NJ, Boyd RD, Faber JJ. Filtration of water from mother to conceptus via paths independent of fetal placental circulation in sheep. J Physiol 431: 1–10, 1990. doi: 10.1113/jphysiol.1990.sp018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DF, Borst CG, Faber JJ. Excess extrafetal fluid without demonstrable changes in placental concentration gradients after week-long infusions of angiotensin into fetal lambs. Eur J Obstet Gynecol Reprod Biol 63: 175–179, 1995. doi: 10.1016/0301-2115(95)02232-5. [DOI] [PubMed] [Google Scholar]
- 8.Anderson D, Yang Q, Hohimer A, Faber J, Giraud G, Davis L. Intramembranous absorption rate is unaffected by changes in amniotic fluid composition. Am J Physiol Renal Physiol 288: F964–F968, 2005. doi: 10.1152/ajprenal.00407.2004. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DF, Jonker SS, Louey S, Cheung CY, Brace RA. Regulation of intramembranous absorption and amniotic fluid volume by constituents in fetal sheep urine. Am J Physiol Regul Integr Comp Physiol 305: R506–R511, 2013. doi: 10.1152/ajpregu.00175.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG. Regulation of amniotic fluid volume. Placenta 28: 824–832, 2007. doi: 10.1016/j.placenta.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Bishop VS, Stone HL, Guyton AC. Cardiac function curves in conscious dogs. Am J Physiol 207: 677–682, 1964. doi: 10.1152/ajplegacy.1964.207.3.677. [DOI] [PubMed] [Google Scholar]
- 12.Brace RA. Effects of outflow pressure on fetal lymph flow. Am J Obstet Gynecol 160: 494–497, 1989. doi: 10.1016/0002-9378(89)90479-1. [DOI] [PubMed] [Google Scholar]
- 13.Brace RA. Fetal blood volume, urine flow, swallowing, and amniotic fluid volume responses to long-term intravascular infusions of saline. Am J Obstet Gynecol 161: 1049–1054, 1989. doi: 10.1016/0002-9378(89)90782-5. [DOI] [PubMed] [Google Scholar]
- 14.Brace RA. Fluid distribution in the fetus and neonate. In: Fetal and Neonatal Physiology, edited by Polin R, Fox W. Philadelphia, PA: Saunders, 1991, p. 1288–1298. [Google Scholar]
- 15.Brace RA. Progress toward understanding the regulation of amniotic fluid volume: water and solute fluxes in and through the fetal membranes. Placenta 16: 1–18, 1995. doi: 10.1016/0143-4004(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 16.Brace RA, Valenzuela GJ. Effects of outflow pressure and vascular volume loading on thoracic duct lymph flow in adult sheep. Am J Physiol Regul Integr Comp Physiol 258: R240–R244, 1990. doi: 10.1152/ajpregu.1990.258.1.R240. [DOI] [PubMed] [Google Scholar]
- 17.Brace RA, Wlodek ME, Cock ML, Harding R. Swallowing of lung liquid and amniotic fluid by the ovine fetus under normoxic and hypoxic conditions. Am J Obstet Gynecol 171: 764–770, 1994. doi: 10.1016/0002-9378(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 18.Brace RA, Vermin ML, Huijssoon E. Regulation of amniotic fluid volume: intramembranous solute and volume fluxes in late gestation fetal sheep. Am J Obstet Gynecol 191: 837–846, 2004. doi: 10.1016/j.ajog.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Brace RA, Cheung CY. Amniotic fluid volume responses to amnio-infusion of amniotic fluid versus lactated Ringer’s solution in fetal sheep. J Soc Gynecol Investig 11: 363–368, 2004. doi: 10.1016/j.jsgi.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Brace RA, Cheung CY. Pre-delivery changes in amniotic fluid volume and composition in sheep. J Soc Gynecol Investig 12: 396–401, 2005. doi: 10.1016/j.jsgi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Brace RA, Cheung CY. Regulation of amniotic fluid volume: evolving concepts. Adv Exp Med Biol 814: 49–68, 2014. doi: 10.1007/978-1-4939-1031-1_5. [DOI] [PubMed] [Google Scholar]
- 22.Brace RA, Anderson DF, Cheung CY. Fetal swallowing as a protective mechanism against oligohydramnios and polyhydramnios in late gestation sheep. Reprod Sci 20: 326–330, 2013. doi: 10.1177/1933719112453510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brace RA, Anderson DF, Cheung CY. Regulation of amniotic fluid volume: mathematical model based on intramembranous transport mechanisms. Am J Physiol Regul Integr Comp Physiol 307: R1260–R1273, 2014. doi: 10.1152/ajpregu.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brace RA, Cheung CY, Anderson DF. Inhibitor of intramembranous absorption in ovine amniotic fluid. Am J Physiol Regul Integr Comp Physiol 306: R185–R189, 2014. doi: 10.1152/ajpregu.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brace RA, Anderson DF, Cheung CY. Ovine fetal swallowing responses to polyhydramnios. Physiol Rep 2: e00279, 2014. doi: 10.1002/phy2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardwell MS. Polyhydramnios: a review. Obstet Gynecol Surv 42: 612–617, 1987. doi: 10.1097/00006254-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Carson GD, Bolla JD, Challis JR. The availability of cortisol in amniotic fluid to the fetus and chorionic and amniotic membranes. Endocrinology 104: 1053–1058, 1979. doi: 10.1210/endo-104-4-1053. [DOI] [PubMed] [Google Scholar]
- 28.Cheung CY. Vascular endothelial growth factor activation of intramembranous absorption: a critical pathway for amniotic fluid volume regulation. J Soc Gynecol Investig 11: 63–74, 2004. doi: 10.1016/j.jsgi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Cheung CY, Brace RA. Amniotic fluid volume and composition in mouse pregnancy. J Soc Gynecol Investig 12: 558–562, 2005. doi: 10.1016/j.jsgi.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CY, Brace RA. Hypoxia modulation of caveolin-1 and vascular endothelial growth factor in ovine fetal membranes. Reprod Sci 15: 469–476, 2008. doi: 10.1177/1933719107312561. [DOI] [PubMed] [Google Scholar]
- 31.Cheung CY, Beardall MK, Anderson DF, Brace RA. Prostaglandin E2 regulation of amnion cell vascular endothelial growth factor expression: relationship with intramembranous absorption rate in fetal sheep. Am J Physiol Regul Integr Comp Physiol 307: R354–R360, 2014. doi: 10.1152/ajpregu.00070.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung CY, Anderson DF, Brace RA. Aquaporins in ovine amnion: responses to altered amniotic fluid volumes and intramembranous absorption rates. Physiol Rep 4: e12868, 2016. doi: 10.14814/phy2.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung CY, Anderson DF, Brace RA. Transport-associated pathway responses in ovine fetal membranes to changes in amniotic fluid dynamics. Physiol Rep 5: e13455, 2017. doi: 10.14814/phy2.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran MA, Nijland MJ, Mann SE, Ross MG. Human amniotic fluid mathematical model: determination and effect of intramembranous sodium flux. Am J Obstet Gynecol 178: 484–490, 1998. doi: 10.1016/S0002-9378(98)70425-9. [DOI] [PubMed] [Google Scholar]
- 35.Daneshmand SS, Cheung CY, Brace RA. Regulation of amniotic fluid volume by intramembranous absorption in sheep: role of passive permeability and vascular endothelial growth factor. Am J Obstet Gynecol 188: 786–793, 2003. doi: 10.1067/mob.2003.160. [DOI] [PubMed] [Google Scholar]
- 36.De Luca G, Olivieri F, Melotti G, Aiello G, Lubrano L, Boner AL. Fetal and early postnatal life roots of asthma. J Matern Fetal Neonatal Med 23, Suppl 3: 80–83, 2010. doi: 10.3109/14767058.2010.509931. [DOI] [PubMed] [Google Scholar]
- 37.Desai M, Ladella S, Ross MG. Reversal of pregnancy-mediated plasma hypotonicity in the near-term rat. J Matern Fetal Neonatal Med 13: 197–202, 2003. doi: 10.1080/jmf.13.3.197.202. [DOI] [PubMed] [Google Scholar]
- 38.Devane GW, Naden RP, Porter JC, Rosenfeld CR. Mechanism of arginine vasopressin release in the sheep fetus. Pediatr Res 16: 504–506, 1982. doi: 10.1203/00006450-198206000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Faber JJ, Thornburg KL. The forces that drive inert solutes and water across the epitheliochorial placentae of the sheep and the goat and the haemochorial placentae of the rabbit and the guinea pig. In: Placental Transfer: Methods and Interpretations, edited by Young M, Boyd RD, Longo LL, Telegdy G. London: Saunders, 1981, p. 203–214. [Google Scholar]
- 40.Faber JJ, Anderson DF. Hydrops fetalis in nephrectomized fetal lambs infused with angiotensin I. Am J Physiol Regul Integr Comp Physiol 267: R1522–R1527, 1994. doi: 10.1152/ajpregu.1994.267.6.R1522. [DOI] [PubMed] [Google Scholar]
- 41.Faber JJ, Anderson DF. Angiotensin mediated interaction of fetal kidney and placenta in the control of fetal arterial pressure and its role in hydrops fetalis. Placenta 18: 313–326, 1997. doi: 10.1016/S0143-4004(97)80066-5. [DOI] [PubMed] [Google Scholar]
- 42.Faber JJ, Anderson DF. Regulatory response of intramembranous absorption of amniotic fluid to infusion of exogenous fluid in sheep. Am J Physiol Regul Integr Comp Physiol 277: R236–R242, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Faber JJ, Anderson DF. Absorption of amniotic fluid by amniochorion in sheep. Am J Physiol Heart Circ Physiol 282: H850–H854, 2002. doi: 10.1152/ajpheart.00746.2001. [DOI] [PubMed] [Google Scholar]
- 44.Faber J, Anderson D, Hohimer R, Yang Q, Giraud G, Davis L. Function curve of the membranes that regulate amniotic fluid volume in sheep. Am J Physiol Heart Circ Physiol 289: H146–H150, 2005. doi: 10.1152/ajpheart.01284.2004. [DOI] [PubMed] [Google Scholar]
- 45.Faber JJ, Brace RA, Davis LE, Anderson DF. Ovine amniotic fluid volume response to intra-amniotic balloon filling. Placenta 30: 201–202, 2009. doi: 10.1016/j.placenta.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flack NJ, Sepulveda W, Bower S, Fisk NM. Acute maternal hydration in third-trimester oligohydramnios: effects on amniotic fluid volume, uteroplacental perfusion, and fetal blood flow and urine output. Am J Obstet Gynecol 173: 1186–1191, 1995. doi: 10.1016/0002-9378(95)91350-5. [DOI] [PubMed] [Google Scholar]
- 47.Fujino Y, Agnew CL, Schreyer P, Ervin MG, Sherman DJ, Ross MG. Amniotic fluid volume response to esophageal occlusion in fetal sheep. Am J Obstet Gynecol 165: 1620–1626, 1991. doi: 10.1016/0002-9378(91)90005-C. [DOI] [PubMed] [Google Scholar]
- 48.Gagnon R, Harding R, Brace RA. Amniotic fluid and fetal urinary responses to severe placental insufficiency in sheep. Am J Obstet Gynecol 186: 1076–1084, 2002. doi: 10.1067/mob.2002.122291. [DOI] [PubMed] [Google Scholar]
- 49.Gesteland KM, Anderson DF, Davis LE, Robertson P, Faber JJ, Brace RA. Intramembranous solute and water fluxes during high intramembranous absorption rates in fetal sheep with and without lung liquid diversion. Am J Obstet Gynecol 201: 85.e1–85.e6, 2009. doi: 10.1016/j.ajog.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilbert WM, Brace RA. Increase in fetal hydration during long-term intraamniotic isotonic saline infusion. Am J Obstet Gynecol 159: 1413–1417, 1988. doi: 10.1016/0002-9378(88)90566-2. [DOI] [PubMed] [Google Scholar]
- 51.Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 74: 748–754, 1989. [PubMed] [Google Scholar]
- 52.Gilbert WM, Cheung CY, Brace RA. Rapid intramembranous absorption into the fetal circulation of arginine vasopressin injected intraamniotically. Am J Obstet Gynecol 164: 1013–1018, 1991. doi: 10.1016/0002-9378(91)90576-D. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert WM, Newman PS, Brace RA. Potential route for fetal therapy: intramembranous absorption of intraamniotically injected furosemide. Am J Obstet Gynecol 172: 1471–1476, 1995. doi: 10.1016/0002-9378(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert WM, Eby-Wilkens E, Tarantal AF. The missing link in rhesus monkey amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 89: 462–465, 1997. doi: 10.1016/S0029-7844(96)00509-1. [DOI] [PubMed] [Google Scholar]
- 55.Gizzo S, Noventa M, Vitagliano A, Dall’Asta A, D’Antona D, Aldrich CJ, Quaranta M, Frusca T, Patrelli TS. An update on maternal hydration strategies for amniotic fluid improvement in isolated oligohydramnios and normohydramnios: evidence from a systematic review of literature and meta-analysis. PLoS One 10: e0144334, 2015. doi: 10.1371/journal.pone.0144334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gresham EL, Rankin JH, Makowski EL, Meschia G, Battaglia FC. An evaluation of fetal renal function in a chronic sheep preparation. J Clin Invest 51: 149–156, 1972. doi: 10.1172/JCI106785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guyton AC, Jones CE, Coleman TG. Circulatory Physiology: Cardiac Output and Its Regulation (2nd ed.). Philadelphia, PA: Saunders, 1973. [Google Scholar]
- 58.Hooper SB, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clin Exp Pharmacol Physiol 22: 235–241, 1995. doi: 10.1111/j.1440-1681.1995.tb01988.x. [DOI] [PubMed] [Google Scholar]
- 59.Hill LM, Breckle R, Thomas ML, Fries JK. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol 69: 21–25, 1987. [PubMed] [Google Scholar]
- 60.Jang PR, Brace RA. Amniotic fluid composition changes during urine drainage and tracheoesophageal occlusion in fetal sheep. Am J Obstet Gynecol 167: 1732–1741, 1992. doi: 10.1016/0002-9378(92)91768-6. [DOI] [PubMed] [Google Scholar]
- 61.Jellyman JK, Cheung CY, Brace RA. Amniotic fluid volume responses to esophageal ligation in fetal sheep: contribution of lung liquid. Am J Obstet Gynecol 200: 313.e1–313.e6, 2009. doi: 10.1016/j.ajog.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kilpatrick SJ, Safford KL, Pomeroy T, Hoedt L, Scheerer L, Laros RK. Maternal hydration increases amniotic fluid index. Obstet Gynecol 78: 1098–1102, 1991. [PubMed] [Google Scholar]
- 63.Kullama LK, Agnew CL, Day L, Ervin MG, Ross MG. Ovine fetal swallowing and renal responses to oligohydramnios. Am J Physiol Regul Integr Comp Physiol 266: R972–R978, 1994. doi: 10.1152/ajpregu.1994.266.3.R972. [DOI] [PubMed] [Google Scholar]
- 64.Lakshmanan J, Ross MG. Mechanism(s) of in utero meconium passage. J Perinatol 28, Suppl 3: S8–S13, 2008. doi: 10.1038/jp.2008.144. [DOI] [PubMed] [Google Scholar]
- 65.Liley AW. Disorders of amniotic fluid. In: Pathophysiology of Gestation, edited by Assali NS. New York: Academic, 1972, p. 157–206. [Google Scholar]
- 66.Lingwood BE, Wintour EM. Permeability of ovine amnion and amniochorion to urea and water. Obstet Gynecol 61: 227–232, 1983. [PubMed] [Google Scholar]
- 67.Luo H, Xie A, Hua Y, Wang J, Liu Y, Zhu X. Aquaporin 1 gene deletion affects the amniotic fluid volume and composition as well as the expression of other aquaporin water channels in placenta and fetal membranes. Clin Chim Acta 482: 161–165, 2018. doi: 10.1016/j.cca.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Macharey G, Gissler M, Rahkonen L, Ulander VM, Väisänen-Tommiska M, Nuutila M, Heinonen S. Breech presentation at term and associated obstetric risks factors—a nationwide population based cohort study. Arch Gynecol Obstet 295: 833–838, 2017. doi: 10.1007/s00404-016-4283-7. [DOI] [PubMed] [Google Scholar]
- 69.Mann SE, Nijland MJ, Ross MG. Mathematic modeling of human amniotic fluid dynamics. Am J Obstet Gynecol 175: 937–944, 1996. doi: 10.1016/S0002-9378(96)80028-7. [DOI] [PubMed] [Google Scholar]
- 70.Mann SE, Nijland MJ, Ross MG. Fetal absorption of intra-amniotic aldosterone: effects on urine composition. J Soc Gynecol Investig 6: 252–257, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Mann SE, Lee JJ, Ross MG. Ovine intramembranous pathway permeability: use of solute clearance to determine membrane porosity. J Matern Fetal Med 10: 335–340, 2001. doi: 10.1080/jmf.10.5.335.340-19. [DOI] [PubMed] [Google Scholar]
- 72.Mann SE, Ricke EA, Torres EA, Taylor RN. A novel model of polyhydramnios: amniotic fluid volume is increased in aquaporin 1 knockout mice. Am J Obstet Gynecol 192: 2041–2044, 2005. doi: 10.1016/j.ajog.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 73.Matsumoto LC, Bogic L, Brace RA, Cheung CY. Prolonged hypoxia upregulates vascular endothelial growth factor messenger RNA expression in ovine fetal membranes and placenta. Am J Obstet Gynecol 186: 303–310, 2002. doi: 10.1067/mob.2002.119806. [DOI] [PubMed] [Google Scholar]
- 74.Moritz KM, Tangalakis K, Wintour EM. Renal, hormonal, and cardiovascular responses to chronic angiotensin I infusion in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 272: R1912–R1917, 1997. doi: 10.1152/ajpregu.1997.272.6.R1912. [DOI] [PubMed] [Google Scholar]
- 75.Morris RK, Meller CH, Tamblyn J, Malin GM, Riley RD, Kilby MD, Robson SC, Khan KS. Association and prediction of amniotic fluid measurements for adverse pregnancy outcome: systematic review and meta-analysis. BJOG 121: 686–699, 2014. doi: 10.1111/1471-0528.12589. [DOI] [PubMed] [Google Scholar]
- 76.Moxey-Mims M, Raju TN. Anhydramnios in the setting of renal malformations: the National Institutes of Health Workshop summary. Obstet Gynecol 131: 1069–1079, 2018. doi: 10.1097/AOG.0000000000002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagiub M, Kanaan U, Simon D, Guglani L. Risk factors for development of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review and meta-analysis. Paediatr Respir Rev 23: 27–32, 2017. [DOI] [PubMed] [Google Scholar]
- 78.Olver RE, Strang LB. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol 241: 327–357, 1974. doi: 10.1113/jphysiol.1974.sp010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oosterhof H, Haak MC, Aarnoudse JG. Acute maternal rehydration increases the urine production rate in the near-term human fetus. Am J Obstet Gynecol 183: 226–229, 2000. doi: 10.1016/S0002-9378(00)17852-4. [DOI] [PubMed] [Google Scholar]
- 80.Phelan JP, Martin GI. Polyhydramnios: fetal and neonatal implications. Clin Perinatol 16: 987–994, 1989. doi: 10.1016/S0095-5108(18)30616-X. [DOI] [PubMed] [Google Scholar]
- 81.Powell TL, Brace RA. Fetal fluid responses to long-term 5 M NaCl infusion: where does all the salt go? Am J Physiol Regul Integr Comp Physiol 261: R412–R419, 1991. doi: 10.1152/ajpregu.1991.261.2.R412. [DOI] [PubMed] [Google Scholar]
- 82.Powell TL, Brace RA. Elevated fetal plasma lactate produces polyhydramnios in the sheep. Am J Obstet Gynecol 165: 1595–1607, 1991. doi: 10.1016/0002-9378(91)90002-9. [DOI] [PubMed] [Google Scholar]
- 83.Robertson P, Faber JJ, Brace RA, Louey S, Hohimer AR, Davis LE, Anderson DF. Responses of amniotic fluid volume and its four major flows to lung liquid diversion and amniotic infusion in the ovine fetus. Reprod Sci 16: 88–93, 2009. doi: 10.1177/1933719108324888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross MG, Ervin MG, Rappaport VJ, Youssef A, Leake RD, Fisher DA. Ovine fetal urine contribution to amniotic and allantoic compartments. Biol Neonate 53: 98–104, 1988. doi: 10.1159/000242768. [DOI] [PubMed] [Google Scholar]
- 85.Ross MG, Brace RA. National Institute of Child Health and Development Conference summary: amniotic fluid biology—basic and clinical aspects. J Matern Fetal Med 10: 2–19, 2001. doi: 10.1080/jmf.10.1.2.19. [DOI] [PubMed] [Google Scholar]
- 86.Sasahara J, Ishii K, Umehara N, Oba M, Kiyoshi K, Murakoshi T, Tanemoto T, Ishikawa H, Ichizuka K, Yoshida A, Tanaka K, Ozawa K, Sago H. Significance of oligohydramnios in preterm small-for-gestational-age infants for outcome at 18 months of age. J Obstet Gynaecol Res 42: 1451–1456, 2016. doi: 10.1111/jog.13074. [DOI] [PubMed] [Google Scholar]
- 87.Scheve EJ, Brace RA. Amniotic fluid volume responses to intra-amniotic infusion of lactate in fetal sheep. J Soc Gynecol Investig 7: 96–101, 2000. doi: 10.1177/107155760000700203. [DOI] [PubMed] [Google Scholar]
- 88.Seeds AE. Water dynamics in the amniotic fluid. In: Amniotic Fluid: Research and Clinical Applications, edited by Fairweather DV, Eskes TK. Amsterdam: Excerpta Medica, 1978, p. 51–57. [Google Scholar]
- 89.Seeds AE. Current concepts of amniotic fluid dynamics. Am J Obstet Gynecol 138: 575–586, 1980. doi: 10.1016/0002-9378(80)90289-6. [DOI] [PubMed] [Google Scholar]
- 90.Shandley L, Alcorn D, Wintour EM. Ovine amniotic and allantoic epithelia across gestation. Anat Rec 248: 542–553, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 91.Sharshiner R, Brace RA, Cheung CY. Vesicular uptake of macromolecules by human placental amniotic epithelial cells. Placenta 57: 137–143, 2017. doi: 10.1016/j.placenta.2017.06.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sherer DM, Cullen JB, Thompson HO, Woods JR. Transient oligohydramnios in a severely hypovolemic gravid woman at 35 weeks’ gestation, with fluid reaccumulating immediately after intravenous maternal hydration. Am J Obstet Gynecol 162: 770–771, 1990. doi: 10.1016/0002-9378(90)91006-X. [DOI] [PubMed] [Google Scholar]
- 93.Shrem G, Nagawkar SS, Hallak M, Walfisch A. Isolated oligohydramnios at term as an indication for labor induction: a systematic review and meta-analysis. Fetal Diagn Ther 40: 161–173, 2016. doi: 10.1159/000445948. [DOI] [PubMed] [Google Scholar]
- 94.Schreyer P, Sherman DJ, Ervin MG, Day L, Ross MG. Maternal dehydration: impact on ovine amniotic fluid volume and composition. J Dev Physiol 13: 283–287, 1990. [PubMed] [Google Scholar]
- 95.Sohl BD, Brace RA. Relationship between graded degrees of anemia and amniotic fluid volume in the ovine fetus. Am J Obstet Gynecol 181: 1552–1559, 1999. doi: 10.1016/S0002-9378(99)70403-5. [DOI] [PubMed] [Google Scholar]
- 96.Thurlow RW, Brace RA. Swallowing, urine flow, and amniotic fluid volume responses to prolonged hypoxia in the ovine fetus. Am J Obstet Gynecol 189: 601–608, 2003. doi: 10.1067/S0002-9378(03)00494-0. [DOI] [PubMed] [Google Scholar]
- 97.Tomoda S, Brace RA, Longo LD. Amniotic fluid volume and fetal swallowing rate in sheep. Am J Physiol Regul Integr Comp Physiol 249: R133–R138, 1985. doi: 10.1152/ajpregu.1985.249.1.R133. [DOI] [PubMed] [Google Scholar]
- 98.Tomoda S, Brace RA, Longo LD. Fate of labeled albumin and erythrocytes following injection into amniotic cavity of sheep. Am J Physiol Regul Integr Comp Physiol 251: R781–R786, 1986. doi: 10.1152/ajpregu.1986.251.4.R781. [DOI] [PubMed] [Google Scholar]
- 99.Tomoda S, Brace RA, Longo LD. Amniotic fluid volume regulation: basal volumes and responses to fluid infusion or withdrawal in sheep. Am J Physiol Regul Integr Comp Physiol 252: R380–R387, 1987. doi: 10.1152/ajpregu.1987.252.2.R380. [DOI] [PubMed] [Google Scholar]
- 100.Volante E, Gramellini D, Moretti S, Kaihura C, Bevilacqua G. Alteration of the amniotic fluid and neonatal outcome. Acta Biomed 75, Suppl 1: 71–75, 2004. [PubMed] [Google Scholar]
- 101.Wintour EM, Barnes A, Brown EH, Hardy KJ, Horacek I, McDougall JG, Scoggins BA. Regulation of amniotic fluid volume and composition in the ovine fetus. Obstet Gynecol 52: 689–693, 1978. [PubMed] [Google Scholar]
- 102.Wlodek ME, Challis JR, Patrick J. Urethral and urachal urine output to the amniotic and allantoic sacs in fetal sheep. J Dev Physiol 10: 309–319, 1988. [PubMed] [Google Scholar]
- 103.Woods LL, Brace RA. Fetal blood volume, vascular pressure, and heart rate responses to fetal and maternal hyperosmolality. Am J Physiol Heart Circ Physiol 251: H716–H721, 1986. doi: 10.1152/ajpheart.1986.251.4.H716. [DOI] [PubMed] [Google Scholar]
- 104.Zheng Z, Liu H, Beall M, Ma T, Hao R, Ross MG. Role of aquaporin 1 in fetal fluid homeostasis. J Matern Fetal Neonatal Med 27: 505–510, 2014. doi: 10.3109/14767058.2013.820697. [DOI] [PubMed] [Google Scholar]