Abstract
Modest cold exposures are likely to activate autonomic thermogenic mechanisms due to activation of cutaneous thermal afferents, whereas central thermosensitive neurons set the background tone on which this afferent input is effective. In addition, more prolonged or severe cold exposures that overwhelm cold defense mechanisms would directly activate thermosensitive neurons within the central nervous system. Here, we examined the involvement of the canonical brown adipose tissue (BAT) sympathoexcitatory efferent pathway in the response to direct local cooling of the preoptic area (POA) in urethane-chloralose-anesthetized rats. With skin temperature and core body temperature maintained between 36 and 39°C, cooling POA temperature by ~1–4°C evoked increases in BAT sympathetic nerve activity (SNA), BAT temperature, expired CO2, and heart rate. POA cooling-evoked responses were inhibited by nanoinjections of ionotropic glutamate receptor antagonists or the GABAA receptor agonist muscimol into the median POA or by nanoinjections of ionotropic glutamate receptor antagonists into the dorsomedial hypothalamic nucleus (bilaterally) or into the raphe pallidus nucleus. These results demonstrate that direct cooling of the POA can increase BAT SNA and thermogenesis via the canonical BAT sympathoexcitatory efferent pathway, even in the face of warm thermal input from the skin and body core.
Keywords: anterior hypothalamus, dorsomedial hypothalamus, metabolism, thermoregulation
INTRODUCTION
In homeothermic animals, the central nervous system regulates body temperature (Tcore) within a narrow range that is determined primarily by the neural control of thermoeffector mechanisms (35). Neurons in the preoptic area (POA) and anterior hypothalamus are a fundamental component within the hierarchical organization of the neural circuits controlling thermoeffector activity (27). The discovery of intrinsically thermosensitive neurons within the POA (6, 32) has led to the concept that brain temperature is primarily sensed by such POA neurons and that the discharge of intrinsically thermosensitive neurons in the POA sets the basal tone of the thermoregulatory system upon which other afferent inputs act, including those from cutaneous thermoreceptors. This idea is supported by the consistent demonstration that the local temperature of the POA can affect thermoeffector output (2–4, 14, 15, 17, 18, 26, 34, 38, 40).
Brown adipose tissue (BAT) is the principal contributor to nonshivering thermogenesis in response to cold exposure, at least in rodents (8). Extensive work has defined the neural circuits controlling BAT sympathetic nerve activity (SNA) (28). Surprisingly, whether direct cooling of the POA increases the sympathetic outflow to BAT is unknown. Therefore, we sought to determine whether directly cooling the POA could increase BAT SNA and BAT thermogenesis and whether the neuroanatomical pathways mediating a POA cooling-evoked increase in BAT SNA and BAT thermogenesis might be similar to those responsible for the activation of BAT thermogenesis in response to skin cooling (29).
Since the rediscovery of BAT in adult humans (10, 36, 46, 47), there has been a robust interest in harnessing the activation of BAT for therapeutic purposes. The current work serves as an important foundation for the potential therapeutic approach of POA cooling to treat metabolic syndrome by increasing BAT energy expenditure and thus its consumption of plasma glucose and fatty acid.
METHODS
Experiments were performed in accord with the regulations detailed in the Guide for the Care and Use of Laboratory Animal (8th ed.; National Research Council, National Academies Press, 2010) and were approved by the Animal Care and Use Committee of the Oregon Health and Science University.
Studies were done in 38 male Sprague-Dawley rats (300–375 g). While the rats were under isoflurane anesthesia (3 in 100% O2), the right femoral artery and vein were cannulated for measurements of arterial pressure and systemic drug injections, respectively, and a tracheotomy was performed to insert an endotracheal tube for artificial ventilation. After venous cannulation, animals were transitioned to urethane and α-chloralose anesthesia (750 and 60 mg/kg iv, respectively). Subsequently, rats were mounted in a stereotaxic apparatus (David Kopf Instruments) with a spinal clamp on the T8-T9 vertebral processes and the incisor bar positioned at −12.0 mm. Rats were artificially ventilated (100% O2) and paralyzed with d-tubocurarine (3 mg/kg iv). Expired CO2 was continuously monitored. A water-perfused thermal blanket containing silicone tubing was wrapped around the rat’s shaved trunk from the shoulders to the hips to produce changes in skin temperature (Tskin) by perfusing the blanket with cold or warm water. Thermocouples were fixed into the rectum for measurement of Tcore, into the left interscapular BAT pad for BAT temperature (TBAT), and into the shaved abdominal skin surface under the thermal blanket for Tskin. Craniotomies overlying the target brain regions were performed to permit the insertion of the bilateral POA cooling thermodes (Fig. 1, C and D) of a thermocouple positioned ~0.5 mm caudal to the tip of the silver rod to measure the preoptic area temperature (TPOA) and of the nanoinjection micropipettes.
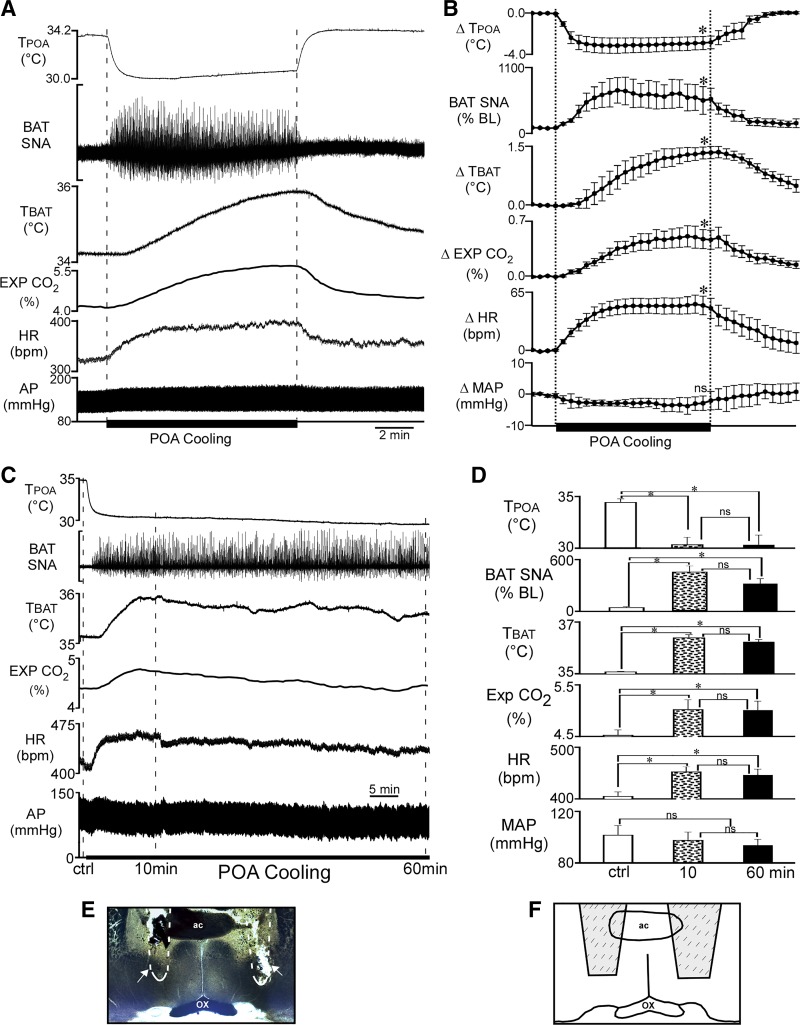
Fig. 1.
Decreasing the temperature of the preoptic area (TPOA) increases brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp CO2), and heart rate (HR). A: representative example of the effect of preoptic area (POA) cooling (between vertical dashed lines) on the recorded variables. B: group data (means ± SE, n = 8). Each time point represents 30-s averages. Statistical comparisons were made between the control precooling level, the cold-evoked level at the 10-min time point of cooling, and the postcooling level at 5 min after terminating the cooling. *P < 0.05, significant difference from the control precooling level. C: representative example of the effect of prolonged POA cooling. D: group data (means ± SE; n = 4). *P < 0.05, significant difference from the control (ctrl) value; ns, not significant. E: representative photomicrograph of the location of the thermodes (area indicated by the outline and arrows). F: schematic illustrating the location of thermodes (shaded region encompasses the entire area in which thermode tracts were found in all rats). The level of the coronal schematic is approximately at bregma. AP, arterial pressure; ac, anterior commissure; ox, optic chiasm.
A custom-built thermode, consisting of two silver rods (0.5-mm diameter and extending ~15 mm from attachment to the Peltier devices) sandwiched between two Peltier devices (TE Technology) and external metal plates to serve as heat sinks, was used to cool the POA bilaterally. With the thermode angled so that the rods were approximately perpendicular to the skull surface, the silver rods were inserted into the POA, bilaterally (coordinates from bregma: +0.5 mm anterior; 1.5 mm lateral and −8.4 mm ventral from dura; Fig. 1, C and D). The Peltier devices were connected to a DC power supply (model 1735A; BK Precision) for passing current (0.2–0.3 Å) to induce decreases in brain temperature. Because of the thermal conductivity of brain tissue, temperature gradients are induced around a thermode (11); therefore, our cooling protocol decreases the local temperature for several millimeters around the thermodes, making it difficult to assess the precise location of the critical temperature-sensitive neurons affected by our cooling protocol. Nonetheless, the area containing thermosensitive neurons has been documented (12) and in the current study the largest responses to brain cooling were obtained when the thermodes were inserted into the POA at a depth of at least 8 mm ventral to dura. In contrast, cooling superficial areas of the brain with the thermodes inserted only a few millimeters ventral to dura yielded little or no activation of BAT SNA and thermogenesis. Therefore, we refer to this cooling protocol as POA cooling.
In the initial POA cooling experiment (Fig. 1, A and B), the control TPOA was lower than Tcore, possibly due to the heat-sink capacity of the thermodes within the POA. Therefore, in all subsequent experiments, a small current of the polarity opposite to that causing cooling was passed to the Peltier device during control periods to maintain TPOA at a stable temperature of 35–36°C, which did not result in any activation of BAT SNA (Figs. 1A and 2A). The Peltier polarity was subsequently reversed to initiate POA cooling.
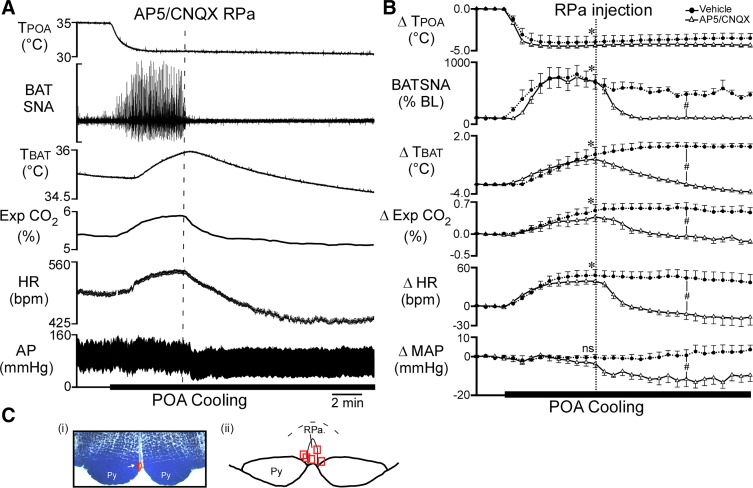
Fig. 2.
Nanoinjection of (2R)-amino-5-phosphonovalericacid (AP5)/6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) into raphe pallidus (RPa) reverses the increase in brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp CO2), and heart rate (HR) evoked by decreasing temperature of the preoptic area (TPOA). A: representative example of the effect of AP5/CNQX injection into RPa (vertical dashed line) on the recorded parameters during local preoptic area (POA) cooling. B: group data (means ± SE, n = 5) for the time course of the responses to AP5/CNQX or saline vehicle injection into RPa during POA cooling. Each time point represents 30-s averages. *P < 0.05, significant difference between the value at the 5th minute of cooling vs. the control precooling value for both the AP5/CNQX trial and the vehicle trial. #P < 0.05, significant difference between the AP5/CNQX-induced change from the cooling-induced peak vs. the change from the cooling-induced peak following vehicle; ns, not significant. Ci: photomicrograph of a representative nanoinjection site in the RPa (red beads indicated by the arrow). Cii: the locations of the nanoinjection sites represented by red squares (n = 5) plotted on a schematic drawing of a partial coronal section at approximately −11.8 mm caudal to bregma. AP, arterial pressure. Py, pyramidal tract.
Bipolar hook electrodes were used to record the activity on small nerve bundles dissected from the ventral surface of the right interscapular BAT pad. BAT SNA was amplified (×10 K, bandpass: 1–300 Hz, CyberAmp 380; Axon Instruments) and digitized (Spike 2; Cambridge Electronic Design) to a hard drive along with all other variables. A continuous measurement (4-s bins) of BAT SNA amplitude was obtained from the autospectra of sequential 4-s segments of raw BAT SNA as the root mean square value (square root of the total power in the 0.1- to 20-Hz frequency band) of the BAT SNA.
To initially verify the viability of the dissected nerve bundles, skin cooling-evoked increases in BAT SNA were tested by perfusing cold water through the thermal blanket or by turning off the heat lamp. After this initial test, to ensure conditions in which there is little to no BAT SNA, Tcore was maintained above 36.5°C, with the use of an infrared heating lamp and by perfusing warm water through the thermal blanket.
Drugs were nanoinjected (60 nl) into the median preoptic nucleus (MnPO; coordinates: from bregma: 0.0 mm rostral, 0.0 lateral, and 6.4 mm ventral to dura), the dorsomedial hypothalamus (DMH; 3.2 mm caudal and 0.5 mm lateral to bregma and 8.2 mm ventral to dura); or the raphe pallidus area (RPa; 3.0 mm caudal to lambda, on the midline, and 9.5 mm ventral from dura). The observation of an increase in BAT SNA and TBAT in response to nanoinjection of N-methyl-d-aspartate (NMDA; 0.2 mM, 60 nl) in MnPO (30) was used to precisely localize the appropriate MnPO site. The following drugs were subsequently nanoinjected into MnPO: (2R)-amino-5-phosphonovalericacid (AP5; 5 mM) combined with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 5 mM, 60 nl) or buffered saline (0.9% sodium chloride sterile, 60 nl). AP5/CNQX (5 mM each in 60 nl) was also injected into DMH (bilateral) and into the RPa during POA cooling. Fluorescent polystyrene microspheres (FluoSpheres F8797, F8801, or F8803; Molecular Probes) were included in the injected drug solutions (1:100 volume dilution of FluoSpheres in the injectate) to allow histological identification of the drug nanoinjection sites.
Histology.
At the end of the experimental procedures, rats were transcardially perfused with 5% formaldehyde. Brains were removed and placed in 30% sucrose solution overnight and sliced (60-μm coronal sections) using a microtome. Drug injection sites and thermode tracts were identified in sections and plotted on schematic drawings.
Data analysis.
Spike2 software (Cambridge Electronic Design) was used to digitize analog signals that were acquired during experiments. Mean arterial pressure (MAP) and heart rate (HR) were obtained from the pulsatile arterial pressure signal. For each experiment, the baseline level of BAT SNA was determined as the mean BAT SNA during a period when the rat was in a warm condition (Tcore and Tskin > 36.5°C) and basal bursting of BAT SNA was absent (i.e., 100% baseline BAT SNA is approximately equal to no ongoing BAT SNA).
For all recorded variables, a repeated measures ANOVA was used to compare the mean control values during the 30-s period before POA cooling, the mean control values during the 30-s periods before nanoinjections or termination of the POA cooling, and the 30-s mean values at a time point 5 min after the nanoinjection or termination of the POA cooling or 10 min after the nanoinjections (for MnPO injections). To assess the drug treatment effects, paired t-tests were performed using StatPlus, comparisons were made between the drug-injected and vehicle-injected trials at each of the time points: 30-s control before cooling, during cooling (30 s before nanoinjection), and 30-s period beginning 5 or 10 min after nanoinjection. Group data are given as mean ± SE. An α-level of 0.05 was used to determine significance.
RESULTS
POA cooling-evoked responses.
Under control conditions in urethane-chloralose-anesthetized rats with Tskin at 36.6 ± 0.5°C and Tcore at 36.4 ± 0.4°C, BAT SNA was low (Fig. 1A; Table 1). Likely due to the intrinsic thermal properties of the thermode to act as a heat sink and the general effect of anesthesia to decrease brain temperature (20), the control TPOA (33.9 ± 0.4°C) was lower than Tcore and Tskin. Passing current to the Peltier device produced a rapid reduction in TPOA, leading to an increase in BAT SNA (Fig. 1, A–D), with activity beginning when TPOA had fallen by −1.3 ± 0.3°C (range: −0.4 to −2.8°C). Cooling the POA (Fig. 1, E and F) also increased TBAT, expired CO2, and HR (Fig. 1). The values of the recorded variables 10 min after beginning POA cooling (Fig. 1A), at a time when TPOA had stabilized at 31.0 ± 0.6°C, are provided in Table 1. Tskin, Tcore, and MAP were not changed during the 10 min of POA cooling (Fig. 1, A and B; Table 1). Termination of the thermode-evoked cooling of the POA returned all measured variables to the control levels within 10 min (Fig. 1, A and B; Table 1). Maintained POA cooling could increase BAT SNA, TBAT, expired CO2, and HR for at least 1 h (Fig. 1, C and D).
Table 1.
Effect of thermode-induced POA cooling
| Pre-POA Cooling | POA Cooling | Post-POA Cooling | |
|---|---|---|---|
| TPOA, °C | 33.9 ± 0.4 | 31.0 ± 0.6* | 33.9 ± 0.4 |
| BAT SNA, %baseline | 100 ± 3 | 575 ± 182* | 213 ± 102 |
| TBAT, °C | 33.7 ± 0.4 | 35.1 ± 0.4* | 34.0 ± 0.5 |
| Exp CO2, % | 4.8 ± 0.3 | 5.3 ± 0.3* | 5.0 ± 0.3 |
| HR, beats/min | 386 ± 12 | 434 ± 10* | 397 ± 8 |
| MAP, mmHg | 101 ± 8 | 100 ± 8 | 102 ± 8 |
| Tskin, °C | 36.6 ± 0.5 | 36.6 ± 0.5 | 36.6 ± 0.5 |
| Tcore, °C | 36.4 ± 0.4 | 36.6 ± 0.1 | 36.6 ± 0.4 |
Values are means ± SE for preoptic area temperature (TPOA), brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp. CO2), heart rate (HR), mean arterial pressure (MAP), core temperature (Tcore), and skin temperature (Tskin) before preoptic area (POA) cooling (pre-POA cooling), during cooling (10 min after beginning of cooling), and 10 min after terminating the cooling (post-POA cooling).
P < 0.05, significantly different from the precooling values.
Antagonism of glutamate receptors in the RPa reverses POA cooling-evoked responses.
The RPa contains the sympathetic premotor neurons for BAT and the glutamatergic activation of these neurons is necessary for many stimuli that increase BAT SNA (23, 29). We determined whether the glutamatergic activation of neurons in the RPa is necessary for the responses evoked by POA cooling by injecting a cocktail of the ionotropic glutamate receptor antagonists AP5 and CNQX into the RPa (Fig. 2C) during the thermode-evoked cooling of the POA. With Tskin and Tcore initially above 37°C, reducing TPOA by 4–5°C increased BAT SNA, TBAT, expired CO2, and HR (Fig. 2, A and B; Table 2). After 5 min of cooling, nanoinjection of AP5/CNQX into the RPa rapidly and completely reversed the increases in BAT SNA, TBAT, expired CO2, and HR evoked by cooling the POA (Fig. 2B; Table 2). AP5/CNQX in the RPa also decreased MAP below the control level before POA cooling (Fig. 2B). In contrast, saline vehicle injection in the RPa did not affect the elevated levels of BAT SNA, TBAT, expired CO2, and HR evoked by POA cooling (Fig. 2B; Table 2).
Table 2.
Effect of glutamate receptor blockade in RPa on POA cooling-evoked responses
| Precooling |
POA Cooling |
Postinjection |
||||
|---|---|---|---|---|---|---|
| Vehicle | AP5/CNQX | Vehicle | AP5/CNQX | Vehicle | AP5/CNQX | |
| TPOA | 34.6 ± 0.3 | 36.0 ± 0.0 | 30.3 ± 0.6* | 31.0 ± 0.0* | 30.9 ± 0.5* | 32.0 ± 0.0* |
| BAT SNA, %baseline | 100 ± 0 | 100 ± 11 | 743 ± 1* | 699 ± 128* | 535 ± 1* | 105 ± 19‡ |
| TBAT, °C | 34.6 ± 0.7 | 35.2 ± 0.4 | 35.8 ± 0.8* | 36.2 ± 0.5* | 36.1 ± 0.8* | 35.2 ± 0.4‡ |
| Exp. CO2, % | 4.6 ± 0.2 | 5.0 ± 0.1 | 5.1 ± 0.3* | 5.3 ± 0.1* | 5.0 ± 0.2* | 4.9 ± 0.1‡ |
| HR, beats/min | 418 ± 10 | 431 ± 13 | 468 ± 16* | 470 ± 10* | 453 ± 14* | 419 ± 13‡ |
| MAP, mmHg | 91 ± 6 | 110 ± 10 | 91 ± 7 | 106 ± 10 | 93 ± 8 | 98 ± 11*‡ |
| Tcore, °C | 37.2 ± 0.7 | 37.7 ± 0.4 | 37.1 ± 0.7 | 37.6 ± 0.4 | 37.1 ± 0.7 | 37.4 ± 0.4* |
| Tskin, °C | 37.3 ± 0.7 | 38.0 ± 0.0 | 37.2 ± 0.7 | 37.0 ± 0.0 | 36.9 ± 0.6 | 37.0 ± 0.0* |
Values are means ± SE for preoptic area temperature (TPOA), brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp. CO2), heart rate (HR), mean arterial pressure (MAP), core temperature (Tcore), and skin temperature (Tskin) before preoptic area (POA) cooling, during cooling (5 min after beginning cooling), and 5 min after injection of either saline vehicle or (2R)-amino-5-phosphonovalericacid (AP5)/6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the raphe pallidus (RPa).
P < 0.05, significantly different from the precooling values.
Significantly different from vehicle-treated group.
Antagonism of glutamate receptors in the DMH reverses POA cooling-evoked responses.
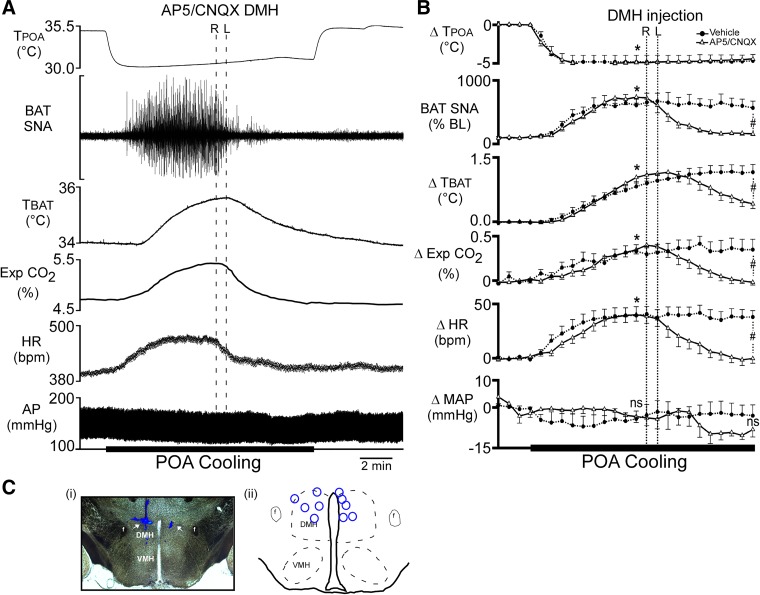
The DMH has been suggested to provide a necessary glutamatergic input to neurons in the RPa for the activation of BAT induced by several stimuli (24, 28). Having demonstrated that a glutamatergic input to the RPa is necessary for the POA cooling-evoked increase in BAT SNA (Fig. 2), we hypothesized that the activation of neurons in the DMH would also be necessary for this response. To determine whether the glutamatergic activation of neurons in the DMH was necessary for the responses evoked by cooling the POA, we injected saline vehicle or a combination of the ionotropic glutamate receptor antagonists AP5 and CNQX into the DMH (Fig. 3C) during the thermode-evoked cooling of the POA. The POA cooling-evoked increases in BAT SNA, TBAT, expired CO2, and HR did not differ between trials in which vehicle or AP5/CNQX were subsequently nanoinjected (Fig. 3B). Bilateral nanoinjections of AP5/CNQX (Fig. 3A) into the DMH completely reversed the increases in BAT SNA evoked by cooling the POA and also decreased TBAT, expired CO2, HR, and MAP from the peak POA cooling-evoked levels (Fig. 3, A and B; Table 3). In contrast, bilateral nanoinjections of vehicle did not affect the elevated levels of BAT SNA, TBAT, expired CO2, and HR evoked by POA cooling (Fig. 3B; Table 3), resulting in significant differences in these variables between the AP5/CNQX-injected and vehicle-injected groups at 5 min after the nanoinjections in the DMH (Fig. 3B; Table 3).
Fig. 3.
Bilateral nanoinjections of (2R)-amino-5-phosphonovalericacid (AP5)/6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) into dorsomedial hypothalamus (DMH) reverse the increases in brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp CO2), and heart rate (HR) evoked by decreasing temperature of the preoptic area (TPOA). A: representative example of the effect of AP5/CNQX injections into DMH (indicated by 2 vertical dashed lines; bilateral injections: R, right side and L, left side) on the recorded parameters during preoptic area (POA) cooling. B: group data (mean ± SE, n = 5) for the time course of the responses to AP5/CNQX or saline vehicle injections into DMH, during preoptic area (POA) cooling. Each time point represents 30 s averages. *P < 0.05, significant difference between the value at the 5th minute of cooling vs. the control precooling value, for both the AP5/CNQX trial and the vehicle trial. #P < 0.05, significant difference between the AP5/CNQX induced change from the cooling-induced peak vs. the change from the cooling-induced peak following vehicle; ns, not significant. Ci: photomicrograph of a representative nanoinjection site in the DMH (arrow indicates blue bead deposits). Cii: a schematic plot of the injection sites in the DMH (blue circles) on a coronal section at approximately −3.0 to −3.3 mm caudal to bregma. AP, arterial pressure. f, fornix.
Table 3.
Effect of glutamate receptor blockade in DMH on POA cooling-evoked responses
| Precooling |
POA Cooling |
Postinjection |
||||
|---|---|---|---|---|---|---|
| Vehicle | AP5/CNQX | Vehicle | AP5/CNQX | Vehicle | AP5/CNQX | |
| TPOA, °C | 35.0 ± 0 | 35.3 ± 0.2 | 30.0 ± 1.0* | 31.2 ± 0.9* | 31.0 ± 1.0* | 31.6 ± 0.7* |
| BAT SNA, %baseline | 100 ± 1 | 100 ± 1 | 610 ± 1.0* | 738 ± 96* | 602 ± 1* | 158 ± 26‡ |
| TBAT, °C | 33.6 ± 0.5 | 34.3 ± 0.3 | 34.9 ± 0.5* | 35.7 ± 0.2* | 35.1 ± 0.5* | 34.7 ± 0.2‡ |
| Exp. CO2, % | 4.8 ± 0.3 | 4.8 ± 0.1 | 5.2 ± 0.3* | 5.2 ± 0.1* | 5.3 ± 0.3* | 4.8 ± 0.1‡ |
| HR, beats/min | 395 ± 13 | 378 ± 19 | 442 ± 19* | 427 ± 24* | 443 ± 16* | 377 ± 20‡ |
| MAP, mmHg | 118 ± 3 | 108 ± 13 | 115 ± 3 | 108 ± 10 | 116 ± 4 | 101 ± 11 |
| Tcore, °C | 36.8 ± 0.5 | 38.0 ± 0.0 | 37 ± 0.5 | 38.0 ± 0.0 | 37.1 ± 0.5 | 38.0 ± 0.0‡ |
| Tskin, °C | 38.0 ± 0.5 | 38.0 ± 0.5 | 38.0 ± 0.5 | 38.0 ± 0.5 | 37.9 ± 0.5 | 37.9 ± 0.5 |
Values are means ± SE for preoptic area temperature (TPOA), brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp. CO2), heart rate (HR), mean arterial pressure (MAP), core temperature (Tcore), and skin temperature (Tskin) before preoptic area (POA) cooling, during cooling (5 min after beginning cooling), and 5 min after injection of either saline vehicle or (2R)-amino-5-phosphonovalericacid (AP5)/6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the dorsomedial hypothalamus (DMH).
P < 0.05, significantly different from the precooling values.
Significantly different from vehicle-treated group.
Antagonism of glutamate receptors in the MnPO reverses POA cooling-evoked responses.
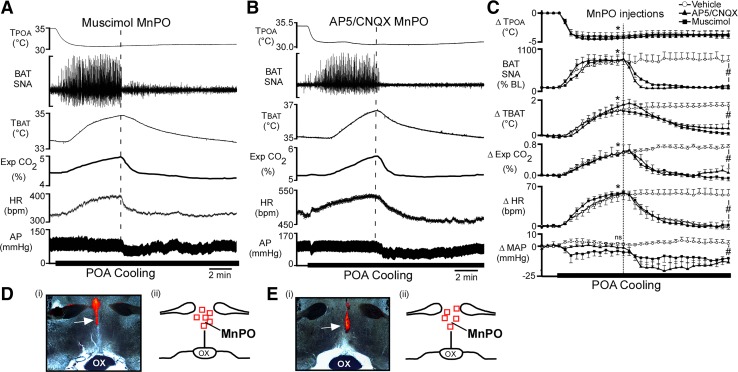
To determine whether the activity of neurons in the MnPO is necessary for the responses evoked by cooling the POA, saline vehicle, the GABAA receptor agonist, muscimol, or a combination of the ionotropic glutamate receptor antagonists AP5 and CNQX was injected into the MnPO (Fig. 4, D and E) during the thermode-evoked cooling of the POA. Under warm conditions, with Tskin and Tcore maintained above 37°C, the POA cooling-evoked increases in BAT SNA, TBAT, expired CO2, and HR did not differ between injection groups (Fig. 4C). Nanoinjection of AP5/CNQX or muscimol into the MnPO completely reversed the increases in BAT SNA TBAT, expired CO2, and HR evoked by POA cooling (Fig. 4; Table 4). In contrast, bilateral nanoinjections of vehicle in the MnPO did not affect the elevated levels of BAT SNA, TBAT, expired CO2, or HR evoked by POA cooling (Fig. 4C). Ten minutes after the nanoinjections in the MnPO, BAT SNA, TBAT, expired CO2, and HR were significantly different between the vehicle-injected and both the AP5/CNQX-injected and muscimol-injected groups (Fig. 4C; Table 4).
Fig. 4.
Nanoinjection of either muscimol or (2R)-amino-5-phosphonovalericacid (AP5)/6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in median preoptic nucleus (MnPO) reversed the increases in brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp CO2), and heart rate (HR) evoked by decreasing temperature of the preoptic area (TPOA). A and B: representative examples of the effects on the recorded variables of muscimol injection (A, vertical dashed line) or AP5/CNQX injection (B, vertical dashed line) into MnPO during preoptic area (POA) cooling. C: group data (means ± SE, n = 5) for the time course of the responses to muscimol, AP5/CNQX, and saline vehicle injections into MnPO during POA cooling. Each time point represents 30-s averages. *P < 0.05, significant difference between the value at the 5th minute of cooling vs. the control precooling value for each group. #P < 0.05, significant difference between the muscimol-induced and AP5/CNQX-induced changes from the cooling-induced peak compared with the vehicle-induced change from the cooling-induced peak; ns, not significant. D and E: photomicrographs (i) and schematic representations (ii) of the nanoinjection sites in MnPO, arrows (i), and red squares (ii) indicate the locations of red bead deposits for muscimol (D) and AP5/CNQX (E). Schematic representations are plotted on a partial coronal section through the POA at ~0.0 to +0.2 mm rostral to bregma. AP, arterial pressure.
Table 4.
Effect of muscimol and AP5/CNQX in MnPO on POA cooling-evoked response
| Precooling |
POA Cooling |
Postinjection |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Muscimol | AP5/CNQX | Vehicle | Muscimol | AP5/CNQX | Vehicle | Muscimol | AP5/CNQX | |
| TPOA, °C | 35.0 ± 0.1 | 35.1 ± 0.1 | 35.5 ± 0.2 | 30.9 ± 0.0* | 30.7 ± 0.7* | 31.9 ± 0.6* | 31.1 ± 0.1* | 31.4 ± 0.7* | 32.1 ± 0.6* |
| BAT SNA, %baseline | 107 ± 9 | 100 ± 0.0 | 100 ± 6 | 755 ± 157* | 801 ± 1.0* | 811 ± 104* | 934 ± 210* | 100 ± 0.0‡ | 106 ± 16‡ |
| TBAT, °C | 35.3 ± 0.1 | 35.4 ± 0.3 | 35.4 ± 0.3 | 36.8 ± 0.2* | 37.2 ± 0.3* | 37.2 ± 0.3* | 37.0 ± 0.1* | 35.3 ± 0.3*‡ | 35.8 ± 0.3‡ |
| Exp. CO2, % | 5.0 ± 0.1 | 5.1 ± 0.2 | 4.7 ± 0.2 | 5.5 ± 0.1* | 5.6 ± 0.2* | 5.4 ± 0.2* | 5.6 ± 0.1* | 4.9 ± 0.2‡ | 4.7 ± 0.2‡ |
| HR, beats/min | 450 ± 8 | 372 ± 13 | 423 ± 10 | 505 ± 5* | 428 ± 19* | 474 ± 9* | 507 ± 4.0* | 369 ± 15‡ | 427 ± 2‡ |
| MAP, mmHg | 102 ± 3 | 108 ± 10 | 102 ± 5 | 103 ± 2 | 104 ± 11 | 99 ± 5 | 106 ± 4 | 99 ± 11‡ | 83 ± 5*‡ |
| Tcore, °C | 38 ± 0.1 | 37.2 ± 0.2 | 38.2 ± 0.3 | 38.1 ± 0.1 | 37.3 ± 0.2 | 38.3 ± 0.3 | 38.1 ± 0.1 | 37.1 ± 0.2 | 38.1 ± 0.3 |
| Tskin, °C | 38.7 ± 0.2 | 37.9 ± 0.4 | 39.1 ± 0.4 | 38.7 ± 0.2 | 37.9 ± 0.4 | 39.1 ± 0.4 | 38.6 ± 0.2 | 37.6 ± 0.3 | 38.6 ± 0.3 |
Values are means ± SE for preoptic area temperature (TPOA), brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired carbon dioxide (Exp. CO2), heart rate (HR), mean arterial pressure (MAP), core temperature (Tcore), and skin temperature (Tskin) before preoptic area (POA) cooling, during cooling (5 min after beginning cooling), and 10 min after injection of either saline vehicle, muscimol, or (2R)-amino-5-phosphonovalericacid (AP5)/6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the median preoptic nucleus (MnPO).
P < 0.05, significantly different from the precooling values.
Significantly different from vehicle-treated group.
DISCUSSION
A major finding of the current study is that POA cooling increases BAT SNA and BAT thermogenesis through a canonical BAT sympathoexcitatory pathway (27) that includes a glutamatergic excitation of thermogenesis-promoting neurons in the DMH (24) and a glutamatergic excitation of BAT sympathetic premotor neurons in the RPa (9, 23, 29). These experiments also revealed a requirement for glutamatergic activation of neurons in the MnPO in the POA cooling-evoked BAT activation.
Skin cooling-evoked activation of BAT thermogenesis is postulated to arise from a disinhibitory activation of thermogenesis-promoting neurons in the DMH (28, 42). During skin cooling, activation of the cutaneous cold afferent pathway activates neurons in the external lateral subdivision of the lateral parabrachial nucleus (LPBel), which provide a glutamatergic input to neurons in the MnPO (31). These MnPO neurons are hypothesized to inhibit warm-sensitive, DMH-projecting, GABAergic neurons in the medial POA, resulting in BAT activation by disinhibition of thermogenesis-promoting neurons in the DMH (28, 30, 31, 42).
Can this model explain the POA cooling-evoked activation of BAT SNA? If direct local cooling of the POA decreased the discharge of intrinsically warm-sensitive, GABAergic neurons in medial POA, then their inhibitory effect on thermogenesis-promoting neurons in the DMH would be reduced, allowing a predominance of tonic excitatory inputs to activate DMH neurons and produce the increase in BAT activation we observed during local POA cooling. This would be similar to the disinhibition of DMH neurons proposed to be a key step in the activation of BAT thermogenesis during skin cooling (27, 30).
The increases in BAT SNA and BAT thermogenesis evoked by POA cooling require glutamatergic activation of neurons in the RPa, which parallels the requirement for glutamatergic activation of sympathetic premotor neurons for BAT in the RPa for a number of stimuli that increase BAT SNA (9, 23), including skin cooling (29). Although the source of this glutamatergic input to RPa has not been directly demonstrated, neurons in the DMH are a likely candidate. DMH neurons with direct projections to RPa are activated during cooling (22, 50) and stress (19, 37), and activation of glutamatergic neurons in the DMH increases thermogenesis (52).
In turn, a glutamatergic excitation of neurons in the DMH is required for the POA cooling-evoked increase in BAT SNA and BAT thermogenesis, as in the neurocircuitry for febrile activation of BAT (24). As in the case of skin cooling, the source of this excitatory input to DMH neurons responsible for increasing their activity to drive increased BAT thermogenesis during POA cooling remains unidentified. Glutamatergic neurons in the MnPO are a potential candidate. The MnPO contains glutamatergic neurons that project to the DMH (1, 13), and this connection has been implicated in cold-evoked thermogenic responses (13, 45). We found that a glutamatergic drive to an, as yet, unidentified population of MnPO neurons is required for the BAT activation during POA cooling. The potential for this glutamatergic excitation to drive MnPO neurons that excite DMH thermogenesis-promoting neurons invites the speculation that the glutamatergic input to the BAT sympathoexcitatory DMH neurons required for the BAT activation during POA cooling (Fig. 3) arises from a population of glutamatergic neurons in the MnPO that projects to the DMH. At the least, our data establish that the activity of a population of MnPO neurons is required for the POA cooling-evoked increase in BAT thermogenesis, and that this activity is supported primarily by a glutamatergic input. This is not to say that POA cooling activates a glutamatergic input to these MnPO neurons but only that a reduction in their activity by the disfacilitatory effect of blocking their ionotropic glutamatergic receptors eliminates POA cooling-evoked BAT activation.
Although previous studies (16, 17) have suggested that the activation of BAT thermogenesis in response to POA cooling requires activation of neurons in the ventromedial hypothalamus (VMH), the results of these earlier studies are uninterpretable since they involved large injections (1 μl) of lidocaine into the VMH, which not only blocked both neuronal action potentials and conduction in axons of passage but also likely diffused dorsally to the DMH. DiMicco and colleagues (51) have made similar arguments in establishing the role of DMH neurons, rather than those in VMH, in the febrile responses mediated by the POA. Surprisingly, whether a glutamatergic input to the DMH is also required for skin cooling-evoked activation of BAT has yet to be demonstrated.
As in the case of skin cooling (31), we found that a glutamatergic activation of a population of neurons in the MnPO is necessary for the POA cooling-evoked increases in BAT SNA and BAT thermogenesis. Glutamate receptor activation in the MnPO is also necessary for the increase in BAT SNA evoked by skin cooling (31). Neurons in the LPBel are an unlikely source of this excitation of MnPO neurons since Tskin and Tcore were maintained at levels that are above those expected to activate cold responsive neurons in the LPBel (31). Thus, if local POA cooling activated BAT SNA and BAT thermogenesis while LPBel neurons were firing at a low rate, it seems unlikely that their glutamatergic input to neurons in the MnPO would play a significant role in the POA cooling-evoked activation of BAT thermogenesis. The source(s) of the critical, glutamatergic drive to MnPO neurons remains to be determined.
In the current studies, the largest responses to brain cooling were obtained when the thermodes were inserted into the POA at a depth of at least 8 mm ventral to dura. In contrast, cooling superficial areas of the brain with the thermodes inserted only a few millimeters ventral to dura yielded little or no activation of BAT SNA and thermogenesis. These data are consistent with the well-established importance of the POA and anterior hypothalamus in thermoregulatory responses (27). Since our cooling protocol decreased the local temperature of the brain tissue for several millimeters around the thermodes, we cannot specify the precise location of the temperature-sensitive neurons that are critical for mediating the effects on BAT thermogenesis that we observed during POA cooling. In addition, the TPOA threshold data in the current studies suggest that the mechanism responsible for these effects is engaged with small changes (as little as −0.4°C) in TPOA; however, since our thermocouple measuring TPOA was positioned ~0.5 mm distal to the thermode, the threshold change in TPOA likely underestimates the change occurring in the immediate vicinity of the thermode. The fact that the TPOA was continuously changing during the assessment of the threshold also limits our ability to determine a precise TPOA threshold for BAT activation. The molecular mechanisms responsible for the temperature sensitivity of POA neurons continue to be studied (5, 21, 41), and the precise contributions of these or other central thermosensitive mechanisms (including the possibility of a Q10 effect decreasing the tonic activity of neurons) to the cooling responses we observed must await further studies.
Brain temperature does not decrease during short-term exposure to cold ambient temperature (7); therefore, the typical physiological responses to moderate cold exposure are not likely to be driven by changes in POA temperature. Prolonged or more severe cold exposures that overwhelm counter-regulatory cold defense mechanisms can lead to decreases in brain temperature sufficient to activate central thermoreceptors in the POA. Hibernation and torpor are accompanied by significant reductions in brain temperature. The blockade of cold-evoked thermogenesis that characterizes these metabolism-reducing states is also likely to involve abrogation (43) of the POA cooling effects on thermogenesis we describe here. Similarly, the hypothermia in sepsis, modeled by administration of high doses of LPS (48), may also involve a marked reduction in the effectiveness of POA cooling to drive thermogenesis.
In full disclosure, these whole animal electrophysiological studies were conducted in anesthetized, paralyzed, artificially ventilated rats in which environmental conditions (e.g., ventilator gases) and certain physiological variables (e.g., skin temperature, respiratory rate, muscle contraction) were precisely controlled. This model was chosen because it permits the recording of BAT SNA during manipulations of the activity of populations of neurons in restricted brain regions, thereby allowing an assessment of their contribution to BAT thermoeffector responses during the delivery of precisely defined stimuli, in this case, preoptic cooling. Although it is obvious that anesthesia will have some effect on all brain functions and that a paralyzed rat will not execute respiratory responses or behaviors, beyond that, we cannot directly assess how, if at all, these factors might have affected the outcomes of our experiments. By comparison, studies conducted in awake, free-behaving animals allow for assessments of thermoeffector activity in the absence of anesthesia or paralysis and are necessary when studying thermoregulatory behaviors, including activity thermogenesis, a cold-defense paradigm prevalent in mice that involves the generation of heat through increased movement. Because only end-organ responses (e.g., BAT temperature) are measured in such studies, it can be difficult to assess indirect effects of an experimental manipulation on thermoeffector activity. For instance, the interpretation of such data may be confounded by changes in the hormonal control of tissue (e.g., BAT) function or by changes in the activity of the monitored thermoeffector that are secondary to the physiological effects that the stimulation/manipulation produces in another tissue (e.g., stress-induced increase in BAT temperature elicited by the animal’s perceptual awareness of the manipulation; or a change in thermogenesis resulting from a stimulus-evoked effect on respiration or on cutaneous blood flow). Thus there are advantages and drawbacks to each of these two broad categories of experimental paradigms, and, when viewed from an unbiased perspective, the results of both approaches provide complimentary opportunities to increase our understanding of central thermoregulation. Specifically, with regard to the data presented here, we are not aware of any experiments conducted in awake, behaving animals that have yielded results that contradict any of those in the current study.
Perspectives and Significance
Since the rediscovery of BAT in adult humans (10, 36, 46, 47), and especially in light of the observations of impaired activation of this tissue in obesity (10, 46), there has been a resurgence of interest in harnessing the activation of BAT to elevate metabolism of adipose tissue for therapeutic purposes (33, 39, 44, 49). Similar to the impairment of cold-induced activation of BAT in obese adult humans (10, 46), skin and core cooling-evoked activation of BAT SNA is attenuated in rats maintained on a high-fat diet (25). The current studies demonstrate that robust increases in BAT SNA and BAT thermogenesis and BAT metabolism can be elicited from local cooling in the POA even in the face of warm thermal inputs from the skin and the body core. Future studies will be necessary to determine whether the impaired activation of BAT in obesity can also be overcome by local cooling in the POA, thus demonstrating the potential for this approach to be used to activate BAT as a therapy for obesity, type 2 diabetes, and other metabolic diseases.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-091066 (to S. F. Morrison) and R01-DK-112198 (to C. J. Madden).
DISCLOSURES
Portions of this work were funded by a Sponsored Research Agreement with CereMod, Inc. (SRA-17-116). K. J. Burchiel, C. J. Madden, and S. F. Morrison have a US patent (US 9,604,060 B2) related to this work. Oregon Health & Science University (OHSU), K. J. Burchiel., C. J. Madden, and S. F. Morrison have a significant financial interest in CereMod, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU.
AUTHOR CONTRIBUTIONS
M.M., C.J.M., K.J.B., and S.F.M. conceived and designed research; M.M. performed experiments; M.M. analyzed data; M.M. prepared figures; M.M. and C.J.M. drafted manuscript; C.J.M. and S.F.M. interpreted results of experiments; C.J.M. and S.F.M. edited and revised manuscript; M.M., C.J.M., K.J.B., and S.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Rubing Xing for histological assistance and to Dr. Virginia Brooks for review of the study results and data analyses.
REFERENCES
- 1.Abbott SB, Machado NL, Geerling JC, Saper CB. Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci 36: 8228–8237, 2016. doi: 10.1523/JNEUROSCI.1244-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin BA, Ingram DL. Effect of heating & cooling the hypothalamus on behavioral thermoregulation in the pig. J Physiol 191: 375–392, 1967. doi: 10.1113/jphysiol.1967.sp008256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banet M, Hensel H. Nonshivering thermogenesis induced by repetitive hypothalamic cooling in the rat. Am J Physiol 230: 522–526, 1976. doi: 10.1152/ajplegacy.1976.230.2.522. [DOI] [PubMed] [Google Scholar]
- 4.Banet M, Hensel H, Liebermann H. The central control of shivering and non-shivering thermogenesis in the rat. J Physiol 283: 569–584, 1978. doi: 10.1113/jphysiol.1978.sp012520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulant JA. Counterpoint: Heat-induced membrane depolarization of hypothalamic neurons: an unlikely mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol 290: R1481–R1484, 2006. doi: 10.1152/ajpregu.00655.2005. [DOI] [PubMed] [Google Scholar]
- 6.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 31, Suppl 5: S157–S161, 2000. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 7.Bratincsák A, Palkovits M. Evidence that peripheral rather than intracranial thermal signals induce thermoregulation. Neuroscience 135: 525–532, 2005. doi: 10.1016/j.neuroscience.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 9.Conceição EP, Madden CJ, Morrison SF. Glycinergic inhibition of BAT sympathetic premotor neurons in rostral raphe pallidus. Am J Physiol Regul Integr Comp Physiol 312: R919–R926, 2017. doi: 10.1152/ajpregu.00551.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean JB, Boulant JA. A diencephalic slice preparation and chamber for studying neuronal thermosensitivity. J Neurosci Methods 23: 225–232, 1988. doi: 10.1016/0165-0270(88)90006-4. [DOI] [PubMed] [Google Scholar]
- 12.Dean JB, Boulant JA. In vitro localization of thermosensitive neurons in the rat diencephalon. Am J Physiol Regul Integr Comp Physiol 257: R57–R64, 1989. doi: 10.1152/ajpregu.1989.257.1.R57. [DOI] [PubMed] [Google Scholar]
- 13.Dimitrov EL, Kim YY, Usdin TB. Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J Neurosci 31: 18166–18179, 2011. doi: 10.1523/JNEUROSCI.2619-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammel HT, Hardy JD, Fusco MM. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol 198: 481–486, 1960. doi: 10.1152/ajplegacy.1960.198.3.481. [DOI] [PubMed] [Google Scholar]
- 15.Hayward JN, Baker MA. Diuretic and thermoregulatory responses to preoptic cooling in the monkey. Am J Physiol 214: 843–850, 1968. doi: 10.1152/ajplegacy.1968.214.4.843. [DOI] [PubMed] [Google Scholar]
- 16.Imai-Matsumura K, Matsumura K, Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn J Physiol 34: 939–943, 1984. doi: 10.2170/jjphysiol.34.939. [DOI] [PubMed] [Google Scholar]
- 17.Imai-Matsumura K, Nakayama T. The central efferent mechanism of brown adipose tissue thermogenesis induced by preoptic cooling. Can J Physiol Pharmacol 65: 1299–1303, 1987. doi: 10.1139/y87-206. [DOI] [PubMed] [Google Scholar]
- 18.Iriki M, Riedel W, Simon E. Regional differentiation of sympathetic activity during hypothalamic heating and cooling in anesthetized rabbits. Pflugers Arch 328: 320–331, 1971. doi: 10.1007/BF00586834. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka N, Hioki H, Kaneko T, Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 20: 346–358, 2014. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav 84: 563–570, 2005. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Hori A, Matsumura K, Hosokawa H. Point-Counterpoint: Heat-induced membrane depolarization of hypothalamic neurons: a putative mechanism of central thermosensitivity. Am J Physiol Regul Integr Comp Physiol 290: R1479–R1480, 2006. doi: 10.1152/ajpregu.00655.2005. [DOI] [PubMed] [Google Scholar]
- 22.Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol 302: R224–R232, 2012. doi: 10.1152/ajpregu.00449.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience 122: 5–15, 2003. doi: 10.1016/S0306-4522(03)00527-X. [DOI] [PubMed] [Google Scholar]
- 24.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 286: R320–R325, 2004. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- 25.Madden CJ, Morrison SF. A high-fat diet impairs cooling-evoked brown adipose tissue activation via a vagal afferent mechanism. Am J Physiol Endocrinol Metab 311: E287–E292, 2016. doi: 10.1152/ajpendo.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martelli D, Luppi M, Cerri M, Tupone D, Mastrotto M, Perez E, Zamboni G, Amici R. The direct cooling of the preoptic-hypothalamic area elicits the release of thyroid stimulating hormone during wakefulness but not during REM sleep. PLoS One 9: e87793, 2014. doi: 10.1371/journal.pone.0087793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SF. Central control of body temperature. F1000 Res 5: 880, 2016. doi: 10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol 4: 1677–1713, 2014. doi: 10.1002/cphy.c140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol 586: 2611–2620, 2008. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science 134: 560–561, 1961. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- 33.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 11: 268–272, 2010. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Thermoregulatory control of sympathetic fibres supplying the rat’s tail. J Physiol 543: 849–858, 2002. doi: 10.1113/jphysiol.2002.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292: R37–R46, 2007. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 36.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci 26: 2228–2238, 2007. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 38.Satinoff E. Behavioral thermoregulation in response to local cooling of the rat brain. Am J Physiol 206: 1389–1394, 1964. [DOI] [PubMed] [Google Scholar]
- 39.Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes 58: 1482–1484, 2009. doi: 10.2337/db09-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smiles KA, Elizondo RS, Barney CC. Sweating responses during changes of hypothalamic temperature in the rhesus monkey. J Appl Physiol 40: 653–657, 1976. doi: 10.1152/jappl.1976.40.5.653. [DOI] [PubMed] [Google Scholar]
- 41.Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353: 1393–1398, 2016. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron 98: 31–48, 2018. doi: 10.1016/j.neuron.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tupone D, Cano G, Morrison SF. Thermoregulatory inversion: a novel thermoregulatory paradigm. Am J Physiol Regul Integr Comp Physiol 312: R779–R786, 2017. doi: 10.1152/ajpregu.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tupone D, Madden CJ, Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Front Neurosci 8: 14, 2014. doi: 10.3389/fnins.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tupone D, Morrison SF. An excitatory projection from median preoptic area to the dorsomedial hypothalamus contributes to the activation BAT thermogenesis. FASEB J 28: 1104, 2014. [Google Scholar]
- 46.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 47.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 48.Wanner SP, Almeida MC, Shimansky YP, Oliveira DL, Eales JR, Coimbra CC, Romanovsky AA. Cold-induced thermogenesis and inflammation-associated cold-seeking behavior are represented by different dorsomedial hypothalamic sites: a three-dimensional functional topography study in conscious rats. J Neurosci 37: 6956–6971, 2017. doi: 10.1523/JNEUROSCI.0100-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittle AJ, López M, Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med 17: 405–411, 2011. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci 29: 11954–11964, 2009. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res 928: 113–125, 2002. doi: 10.1016/S0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 52.Zhao ZD, Yang WZ, Gao C, Fu X, Zhang W, Zhou Q, Chen W, Ni X, Lin JK, Yang J, Xu XH, Shen WL. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci USA 114: 2042–2047, 2017. [Erratum in Proc Natl Acad Sci USA 114: E1755, 2017. https://doi.org/10.1073/pnas.1701881114.] doi: 10.1073/pnas.1616255114. [DOI] [PMC free article] [PubMed] [Google Scholar]