Abstract
Background
Nitrite is reduced by heme-proteins and molybdenum-containing enzymes to form the important signaling molecule nitric oxide (NO), mediating NO signaling. Substantial evidence suggests that deoxygenated hemoglobin within red blood cells (RBCs) is the main erythrocytic protein responsible for mediating nitrite-dependent NO signaling. In other work, infrared and far red light have been shown to have therapeutic potential that some attribute to production of NO. Here we explore whether a combination of nitrite and far red light treatment has an additive effect in NO-dependent processes, and whether this effect is mediated by RBCs.
Methods and results
Using photoacoustic imaging in a rat model as a function of varying inspired oxygen, we found that far red light (660 nm, five min. exposure) and nitrite feeding (three weeks in drinking water at 100 mg/L) each separately increased tissue oxygenation and vessel diameter, and the combined treatment was additive. We also employed inhibition of human platelet activation measured by flow cytometry to assess RBC-dependent nitrite bioactivation and found that far red light dramatically potentiates platelet inhibition by nitrite. Blocking RBC-surface thiols abrogated these effects of nitrite and far-red light. RBC-dependent production of NO was also shown to be enhanced by far red light using a chemiluminescence-based nitric oxide analyzer. In addition, RBC-dependent bioactivation of nitrite led to prolonged lag times for clotting in platelet poor plasma that was enhanced by exposure to far red light.
Conclusions
Our results suggest that nitrite leads to the formation of a photolabile RBC surface thiol-bound species such as an S-nitrosothiol or heme-nitrosyl (NO-bound heme) for which far red light enhances NO signaling. These findings expand our understanding of RBC-mediated NO production from nitrite. This pathway of NO production may have therapeutic potential in several applications including thrombosis, and, thus, warrants further study.
Abbreviations: NO, nitric oxide; RBC, red blood cell; Hb, hemoglobin; MetHb, methemoglobin; CPTIO, 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; FiO2, fraction of inspired oxygen; ADP, Adenosine diphosphate; FITC, Fluorescein, DTNB, 5,5-dithio-bis-(2-nitrobenzoic acid); PBS, phosphate buffered saline; AUC, area under the curve; Hct, hematocrit; BSA, bovine serum albumin; tPA, tissue plasminogen activator; OD, optical density; FR, far red
Keywords: Nitric oxide, Nitrite, Red blood cells, Hemoglobin, Light therapy, Photobiomodulation
Graphical abstract
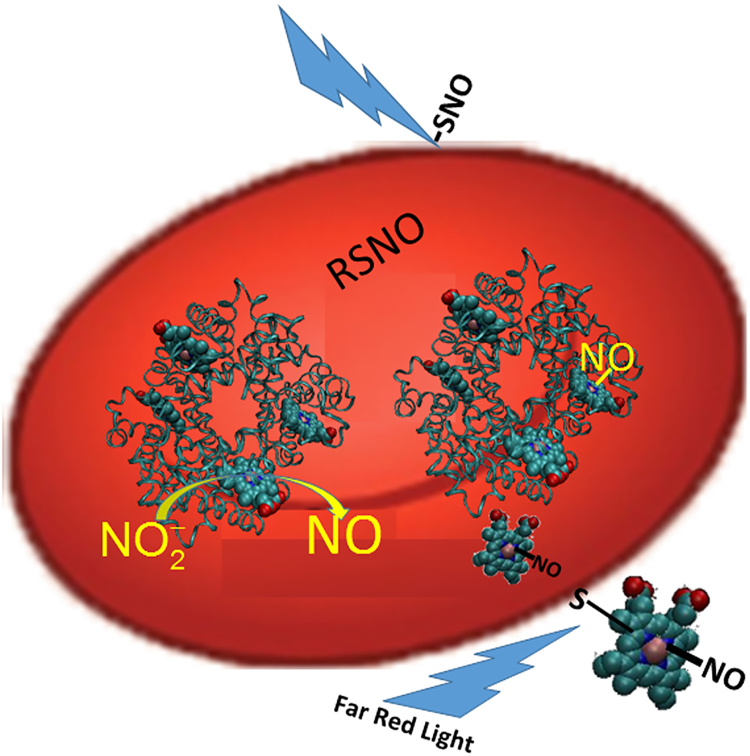
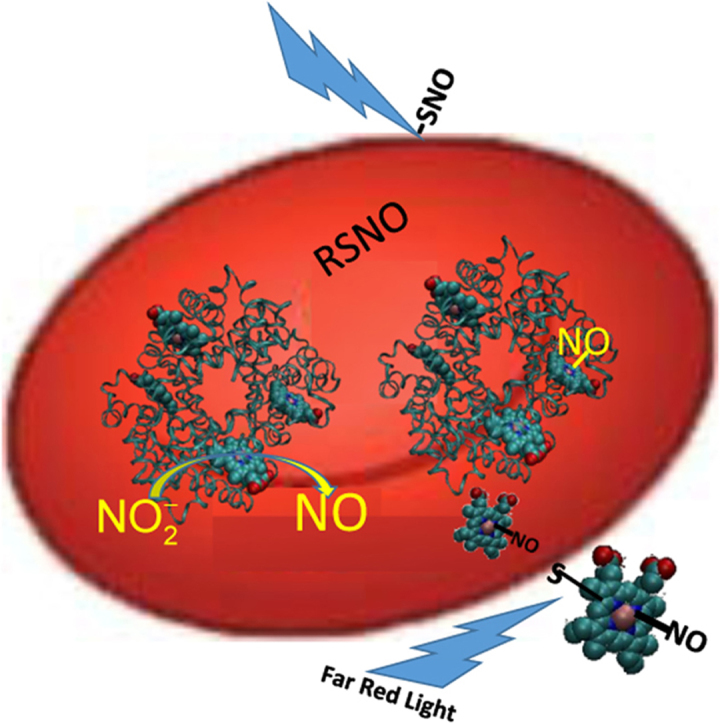
Potential mechanism of RBC-mediated nitrite bioactivation and its potentiation by far red light. Nitrite reacts with deoxygenated Hb to make NO which then binds other vacant hemes or forms a nitrosothiol (RSNO). The nitrosyl-heme or nitrosothiol is exported and binds a surface thiol. Another potential NO species that may form is a DNIC (not shown). The NO congener can then be transported in plasma and interact with platelets and other blood components and this action is potentiated by photolysis using far red light.
Highlights
-
•
Nitrite and far red light increase tissue oxygenation under hypoxia.
-
•
Far red light enhances nitrite-mediated inhibition of platelet activation by red cells.
-
•
Blocking red cell surface thiols abrogates nitrite-mediated effects.
-
•
NO production from nitrite and red cells is enhanced by far red light.
-
•
Lag time in clotting is prolonged by nitrite plus far red light.
1. Introduction
Nitric oxide (NO) is an important signaling molecule that causes vasodilation, inhibits platelet activation, and decreases circulating blood cell adhesion [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Once thought to be biologically inert in human physiology [13], we and others proposed that nitrite serves as a storage pool for nitric oxide that is harnessed under hypoxic conditions. We and others proposed a new function of deoxygenated hemoglobin that targets NO delivery to hypoxic areas where it is needed the most [14], [15]. The reduction of nitrite to NO by deoxygenated, ferrous hemoglobin is well-known and illustrated in Eq. (1) below [16], [17], [18].
| (1) |
where MetHb is the ferric, oxidized form, methemoglobin and Hb is deoxygenated ferrous hemoglobin. The physiological relevance of NO production by hemoglobin is challenged by its rapid reaction with oxygenated hemoglobin (k = 6–8 × 107 M−1 s−1) to form nitrate (Eq. (2)) [19], [20], [21],
| (2) |
However, experiments studying relaxation of aortic rings by nitrite in the presence and absence of red blood cells (RBCs) support the hypothesis that RBCs bioactivate nitrite [14], [22]. Moreover, nitrite has been shown to inhibit platelet activation in the presence, but not absence of RBCs and this action is potentiated in hypoxia [23], [24], [25]. In addition, inhibition of platelet activation by nitrite and RBCs in blunted by the NO scavenger CPTIO [23], [25], strongly supporting the notion that RBCs produce NO from nitrite.
Several pathways have been suggested to resolve the paradox of how NO activity can be exported from the RBC and escape scavenging through dioxygenation (Eq. (2)) that is predicted to prevent NO export [15], [26], [27], [28], [29], [30], [31]. It should also be noted that several other enzymes besides Hb have been proposed as physiological nitrite reductases [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], including some found in RBCs. However, our work confirms the role of Hb as the primary erythrocytic nitrite reductase [45]. Recently, using inhibition of platelet activation as an indicator of RBC-mediated bioactivation of nitrite, we found that surface thiols on the RBCs may participate as stable intermediates in erythrocytic nitrite bioactivation and NO signaling via nitrosation involving N2O3 or other intermediates [25], [26].
Evidence suggests positive effects of near infrared (700–1400 nm) or far-red (600–700 nm) light including increased vasodilation, increased blood flow, and angiogenesis with therapeutic applications in wound healing, tissue generation, reduction of inflammation, and improving cognition [46], [47], [48], [49]. The mechanism for these actions has been attributed to modulation of the redox state of cytochrome c oxidase [49], [50], [51], [52], [53]. However, several investigators have suggested that increased nitric oxide activity is largely responsible [48], [54], [55], [56], [57], [58]. In particular, a few investigators have suggested that the light releases NO from nitrosothiols or other NO containing species [55], [56], [57]. We hypothesize that nitrite bioactivation by RBCs results in production of photolabile NO species on the surface of the RBCs so that the combination of far red light illumination and nitrite administration would result in enhanced NO-dependent processes.
2. Materials and methods
2.1. Materials
Whole blood was collected by venipuncture from volunteers after obtaining informed consent following a protocol approved by the internal review board at Wake Forest University health sciences (IRB##BG02–168). All animal protocols were approved by the institutional Animal care and Use Committee (IACUC #A17–777). All materials were obtained from Sigma Aldrich except as noted otherwise.
3. Methods
3.1. Far red light treatment
A “deep red” LED source (M660L4, Thorlabs, NJ) at a center wavelength of 660 nm was used to illuminate the samples through an aspheric condenser lens (ACL5040U-B, Thorlabs, NJ) and a focusing lens (LA1050-B, Thorlabs, NJ). The setup produced a 1 cm diameter spot on the sample. The incident light power in the sample was measured by a power meter (1815-C, Newport, CA) to be between 65 mW and 560 mW as described in the results section. The power for each experiment was a function of the optical set-up for each experiment.
3.2. Photoacoustic imaging
Photoacoustic imaging was performed as described previously using a Vevo 2100 LAZR system (VisualSonics Inc., Toronto, ON, Canada) [59]. This device captures coregistered B-Mode ultrasound images along with the photoacoustic image that allows for real-time mapping of tissue oxygenation. Male Sprague Dawley rats were either given nitrite containing water (100 mg/L) or control water. On the day of imaging, isoflurane (5% induction; 1.5% maintenance, balance oxygen) was used for anesthesia. The hindlimb in the region of the femoral artery was imaged at different fractions of inspired oxygen (FiO2) lowered stepwise from 1.0 to 0.21, 0.15, and 0.08. Images were taken after a five minute equilibration period at each FiO2. After a complete cycle with no illumination, FiO2 was taken back up to 1.0 and the animals equilibrated for 15 min before measurements were repeated in the presence of light.
3.3. Platelet activation assay
Preparation of platelets and deoxygenated RBCs were performed as described previously [24]. Platelet and RBC mixtures were incubated at room temperature (21 °C) with and without nitrite (10 µM) and with and without far red light 660 nm for five minutes followed by addition of ADP (1 µM) to initiate activation. The mixture was then incubated for another five minutes. FITC labeled PAC-1 and Per-CP labeled CD61 antibodies were added to 10 µL of the sample for an additional 15 min before fixation with 1% buffered formalin. Platelet activation was quantified as percent PAC-1 positive using a BD Bioscience FACS Calibur flow cytometer.
3.4. Chemiluminescence-based NO measurements
NO production from RBCs and nitrite in the presence vs absence of far red light was tested by a chemiluminescence assay using a Nitric Oxide Analyzer (Sievers, GE Analytical Instruments). A three-necked round bottom flask was used as described previously [45]. NO released was measured from the round bottomed flask with 20 ml of deoxygenated phosphate buffer (pH 6.8) containing 20 mM nitrite and injection of 100 µL deoxyRBCs (50% hematocrit (Hct) in PBS, pH 6.8, with or without exposure to far-red light at 660 nm, 65 mW for 1 h. The round bottomed flask was placed near a hot plate; reaction temperature was maintained at 32.5 °C. The sample was continuously, gently stirred with a magnetic stir bar. Total NO released for each treatment was quantified by determining the area under the curve (AUC) by summing up the signal from the NO Analyzer.
3.5. Turbidity assay of fibrinogen polymerization
The plasma clot turbidity and fibrinolysis assay was performed as described previously using lyophilized, platelet-poor, pooled plasma (George King Biomedical Inc) [60]. Plasma (720 µL) was combined with 480 µL of 0.05 M phospholipid (Rossix, AB, Sweden) as well as 480 µL of assay buffer (50 mM Tris-HCl, 140 mM NaCl, 1 mg/ml BSA, pH 7.4) to make the plasma mixture. When the assay was performed with tissue plasminogen activator (tPA), 60 µL of 25 ng/ml tPA and 420 µL of assay buffer was added to the plasma mixture instead of the 480 µL of assay buffer. For the activation mixture, which was prepared separately from the plasma mixture, 200 µL assay buffer, 300 µL 250 mM CaCl2, and 200 µL of 12.5 units/ml human alpha thrombin was used.
RBCs were added after washing whole blood with assay buffer. Both the plasma mixture (1680 µL) and the activation mixture (700 µL) were suspended on top of packed RBC to achieve a 22% Hct. All solution were degassed for at least 30 min. For the first treatment, 100 µM nitrite was added to both the plasma mixture and activation mixture at 240 µL and 100 µL respectively, achieving a final nitrite concentration of 10 μM in both mixtures. For the second treatment, 100 µM nitrite was added in combination with a 10-min exposure to far red light for each sample. In all assays the final Hct was 20% and concentrations of all reagents (calcium, plasma (containing fibrinogen), thrombin) were the same. Prior to turbidity measurements, the RBCs were spun down (15 min at 500 g) and the supernatant was collected and added to a 96-well flat bottom assay plate. Clotting was initiated the instant 80 µL plasma mixture was loaded with 20 µL activation mixture. The activation mixture was added simultaneously to the wells using a multichannel pipet. The final clotting reactions contained 30% plasma, 10 μM phospholipids, 0.5 NIH units/ml thrombin, 15 mM CaCl2, 140 mM NaCl, 15 mM Tris-HCl, pH 7.4.
Immediately after addition of the activation mixture, the plate was transferred to a microplate reader that measured the optical density at 405 nm (or 490 nm depending on amount of hemolysis) every 6–8 s for 3 h. If the clot was formed in the absence of tPA, 15 min total run time was used.
Clotting parameters were defined as described previously [60]. The maximum optical density was found by examining the digitized data and correcting (subtracting) the initial optical density prior to clotting. The clot lysis time was calculated by determining the time from clot formation to clot lysis. The two time points were determined when the optical density (corrected for the initial optical density) was half the maximum at clot formation and at clot lysis. The lag time for clotting was determined objectively as the difference between the time when clotting was initiated and the time when the optical density rose to a level that was 5% more than the initial optical density.
3.6. Temperature
Unless otherwise indicated, all in vitro experiments were performed at room temperature which was measured to be 21 °C. To asses effects of far red light treatment on sample heating we employed a Digi-Sense Dual J-T-E-K thermocouple with a type K thermocouple probe to measure the temperature of the samples after exposure to light. When a temperature increase was discovered, control experiments were performed to rule out heating as a mechanism for observed far red light effects by repeating experiments at the final temperature after light exposure and comparing to room temperature.
3.7. Statistics
When one treatment was performed along with another on the same day with a single variable being altered (example +/- nitrite), a paired, two-tailed t-test was performed. Otherwise, when variables were changed on different days and treatments compared, an un-paired, two tailed t-test was used. When comparing multiple groups ANOVA test was used. Significance was set at p < 0.05.
4. Results
4.1. Far red light potentiates the microvascular effects of nitrite
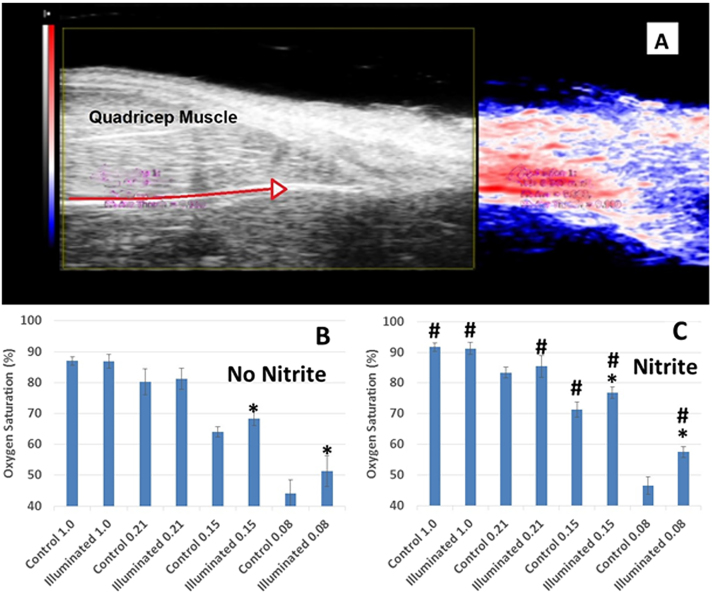
If the combination of far red light illumination and nitrite results in elevated NO bioavailability, then one would expect to observe increased tissue oxygenation and vessel diameters during hypoxia with light and nitrite exposure. We employed a rat hypoxic shock model and photoacoustic imaging to test these effects of nitrite and far red light. Photoacoustic imaging is a non-invasive technique capable of measuring mean microvascular saturation along with structural information in a model where hypoxia is induced by lowering the fraction of inspired oxygen (FiO2) [59]. A representative set of images in shown in Fig. 1A. The image on the left is a standard 2D B-mode ultrasound image and that to the right is the co-registered photoacoustic one that includes the vessel and tissue above it. Red indicates high oxygen saturation while blue corresponds to low oxygen saturation. An intense red color is present at the areas of highest oxygen saturation (in the artery) with areas of lower saturation becoming less red and then blue at distances further from the artery. Imaging and oxygen measurements were conducted under hyperoxia (FiO2 1.0), normoxia (FiO2 0.21), and mild (FiO2 0.15) and extreme hypoxia (FiO2 0.08). One group of animals received nitrite in their drinking water (100 mg/L) while another group drank water without nitrite treatment. In the nitrite untreated group, illumination with far red light resulted in increased tissue oxygenation in extreme hypoxia and mild hypoxia compared to when light was absent (Fig. 1B). This effect of light was also observed in the nitrite-treated animals (Fig. 1C). In addition, nitrite treatment resulted in significantly higher tissue oxygenation than in nitrite untreated animals at all FiO2 levels (with and without illumination) except at 0.21 and 0.08 FiO2 in the absence of light (two blue bars in Fig. 1C that do not have #.) Overall the effect of nitrite was highly significant compared to no nitrite (Fig. 1B,C). The effect was observed at physiological nM concentrations of nitrite, with effects observed at nitrite levels of 0.35 ± 0.19 μM vs 0.23 ± 0.09 μM (p = 0.31, n = 4) and nitrate levels of 13 ± 3 μM vs 9 ± 1 μM (p = 0.07, n = 4). Five minutes of far red light exposure also significantly increased tissue femoral artery diameter (685.4 ± 9.6 µm vs. 718.8 ± 6.3 µm; p = 0.01 (data not shown) in hindlimb tissue during extreme hypoxia in all animals (n = 8, p < 0.05) with similar trends with mild hypoxia. In a two-way ANOVA analysis of the tissue oxygenation, the effects of both nitrite and light were each highly significant (P < 0.015), but the interaction between these two treatments was not significant (P = 0.2). To further explore mechanisms of these treatments, we turned to in vitro studies of platelet activation.
Fig. 1.
Microvascular oxygen saturation measurements obtained from photoacoustic imaging in rats. Oxygen saturation was measured using photoacoustic imaging while rats inhaled different fractions of inspired oxygen (FiO2). (A) Representative images. Left: standard 2D B-mode ultrasound image. Right: co-registered photoacoustic image with setting optimized to the depth of that vessel and tissues above it. The red arrow in the 2D B-mode indicates the course of the femoral artery. Intense red indicates high oxygen saturation; blue indicates low saturation. (B) Effects of far red light (660 nm, 150 mW, 1 cm spot size, 5 min) in control-fed rats. Tissue oxygenation is shown without illumination (blue bars) by far red light adjacent to that with far red light (red bars) for each FiO2. n = 8, *p < 0.05 illuminated vs not illuminated. Data are plotted as mean ± one standard deviation. (C) Effects of far red light in nitrite-fed rats. n = 8, *p < 0.05 illuminated vs not illuminated, #P < 0.05 nitrite vs no nitrite. In two-way Anova, P < 0.0001 nitrite vs no nitrite. Data are plotted as mean ± one standard deviation.
4.2. Far red light potentiates the effect of nitrite on platelet activation
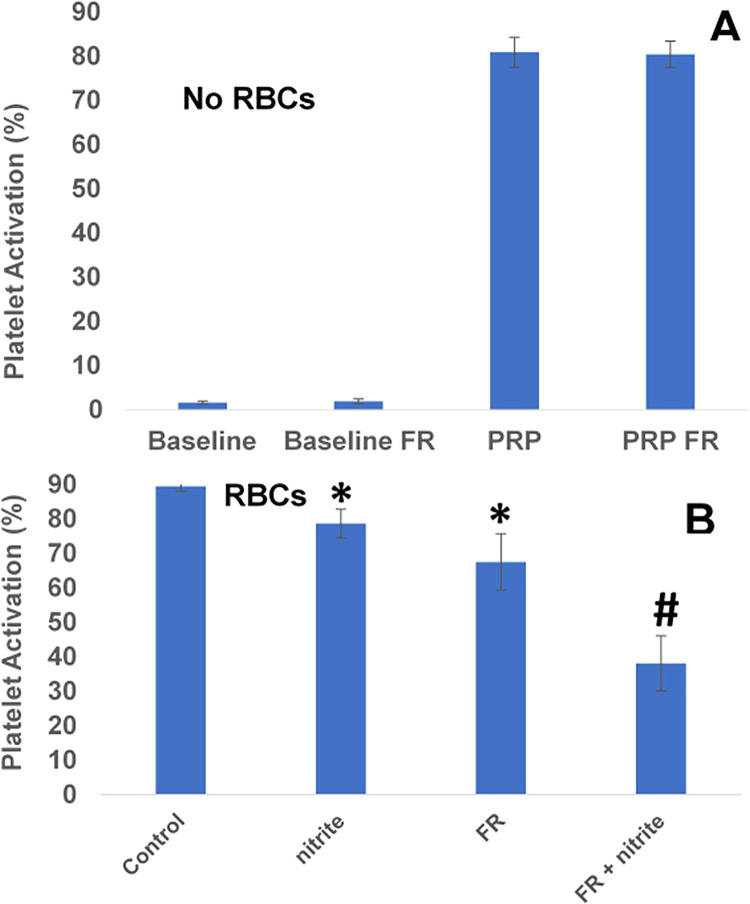
We used inhibition of platelet activation as a measure of nitrite bioactivation by RBCs (Fig. 2). Illumination with far red light (660 nm, 560 mW) for 5 min in the absence of RBCs had no effect on activation in PRP at baseline or after activation using ADP (Fig. 2A). As previously reported, the combination of nitrite (10 μM) and partially deoxygenated RBCs decreased platelet activation (Fig. 2B, p = 0.01, n = 9). In addition, treatment with light alone decreased platelet activation when RBCs were present (Fig. 2B, p < 0.02, n = 9). Inhibition was dramatically enhanced by the combination of illumination and nitrite (Fig. 2B). In a two-way ANOVA analysis of the platelet activation in the presence of RBCs, the effects of both nitrite and light were each highly significant (P < 0.015), but the interaction between these two treatments was not significant (P = 0.095, n = 9). However, if one anomalous trial (where light treatment reduced platelet activation anomalously to 19% and light + nitrite treatment reduced it to 11%) of the nine trials is excluded the interaction between treatments is significant (P = 0.016). A significant interaction term indicates that the two treatments are synergistic (not just additive) and would be consistent with our hypothesis that nitrite treatment forms NO modified, photolabile RBC surface thiols.
Fig. 2.
Red blood cell bioactivation of nitrite is enhanced by far red light. (A) Platelet activation in the absence of RBCs. Platelets in platelet rich plasma (PRP) were activated with 1 μM ADP compared to baseline. Exposure to far red light (FR, 660 nm, 560 mW, 5 min) had no effect on baseline activation or PRP with ADP. n = 9. (B) Platelet activation in the presence of deoxygenated RBCs. Nitrite (10 μM) reduced platelet activation in the presence of deoxygenated RBCs (15% Hct, *P < 0.02, paired t-test, n = 9). Illumination with far red light also reduced platelet activation compared to control (*P < 0.02, paired t-test, n = 9). Data are plotted as mean ± one standard error. The combination of light and nitrite treatments further reduced platelet activation (#P < 0.001, paired t-test FR + nitrite vs FR, n = 9). Data are plotted as mean ± one standard error.
4.3. RBC surface thiols are involved in effects of far red light and nitrite
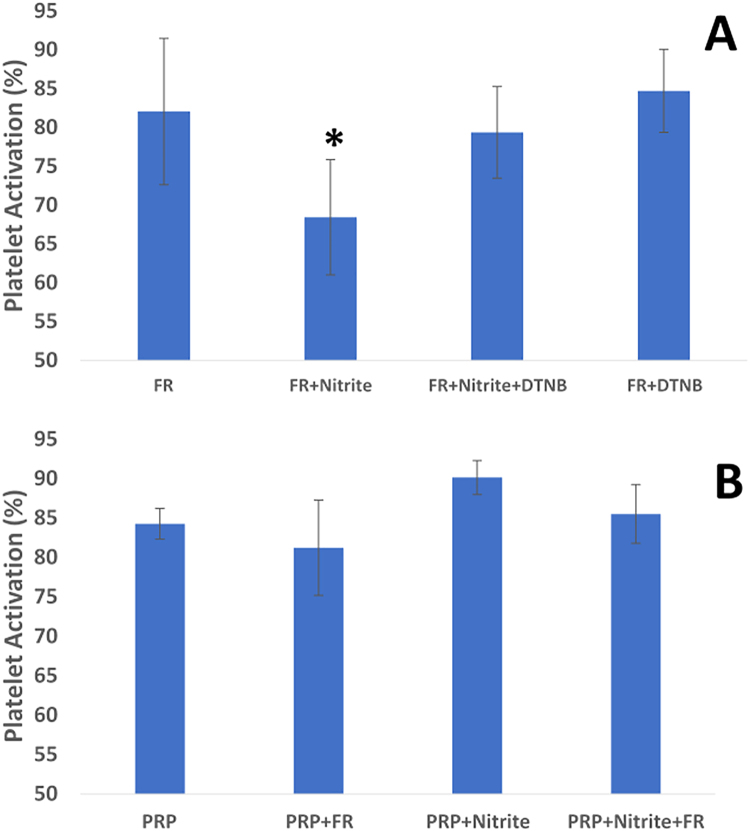
To test whether the combined effects of light and nitrite are dependent on RBC surface thiols, we employed the membrane impermeable, thiol blocking agent DTNB (Fig. 3A) and tested nitrite's ability to inhibit platelet activation. We observed that pretreatment of RBCs with this surface thiol blocking agent abrogated the inhibitory effects of combined nitrite and light treatment. These data suggest that nitrite treatment of RBCs results in a photolabile compound such as a nitrosothiol or thiol bound nitrosyl heme that transduces NO activity and inhibits platelet activation.
Fig. 3.
Mechanistic aspects of effects of nitrite and light. (A) Effect of blocking surface thiols on effects of nitrite and far red light. Platelets were activated with 1 μM ADP yielding 84.2 ± 6.8% activation (bar labeled PRP). Addition of deoxygenated RBCs (deoxyRBC) and five minutes of exposure to far red light (FR, 660 nm, 150 mW) did not significantly affect activation. However, combination of nitrite (10 μM) and light reduced platelet activation. When the RBCs were pre-incubated with DTNB (2.5 mM, one hour), inhibition of platelet activation was abrogated (n = 5, *P < 0.01 for FR+nitrite vs any other condition shown). Data are plotted as mean ± one standard deviation. (B) Effects of light and nitrite on platelet activation in the absence of RBCs. Platelets were activated by 1 μM ADP (PRP). Illumination with far red light (FR, 660 nm, 5 min), 10 μM nitrite, or the combination of the two had no significant effect on platelet activation (n = 3). Data are plotted as mean ± one standard deviation.
To confirm that the effects of nitrite and light are RBC-dependent, we performed additional experiments using PRP to assess whether nitrite and/or light would inhibit platelet activation in the absence of RBCs (Fig. 3B). Consistent with previous reports [23], [24], nitrite had no effect on platelet activation when RBCs were absent. In addition, neither far red light nor the combination of nitrite and far red light had a significant effect on platelet activation in PRP samples when RBCs were absent. Thus, RBCs are necessary for inhibition of platelet activation by nitrite, far red light, and their combination.
One may suggest that the potentiation in inhibition of platelet activation by light is due to heating of the PRP and RBCs which enhances the nitrite reaction. We measured that under the conditions used, illumination with the far red light resulted in an increase in temperature of 5 °C after ten minutes of illumination (from 21 °C to 26 °C). Thus, to examine if temperature was a factor, we measured the effects of nitrite-mediated inhibition of platelet activation in the absence of illumination at controlled temperatures of 21 °C and 26 °C. We found no difference in the percent inhibition of platelet activation at these temperatures ((platelet activation with nitrite – platelet activation without nitrite)/ platelet activation without nitrite): 13% ± 6% at 21 °C vs 13% ± 2% at 26 °C, n = 3, P = 0.46. Thus, heating does not contribute to the observed potentiation of far red light on nitrite-mediated inhibition of platelet activation.
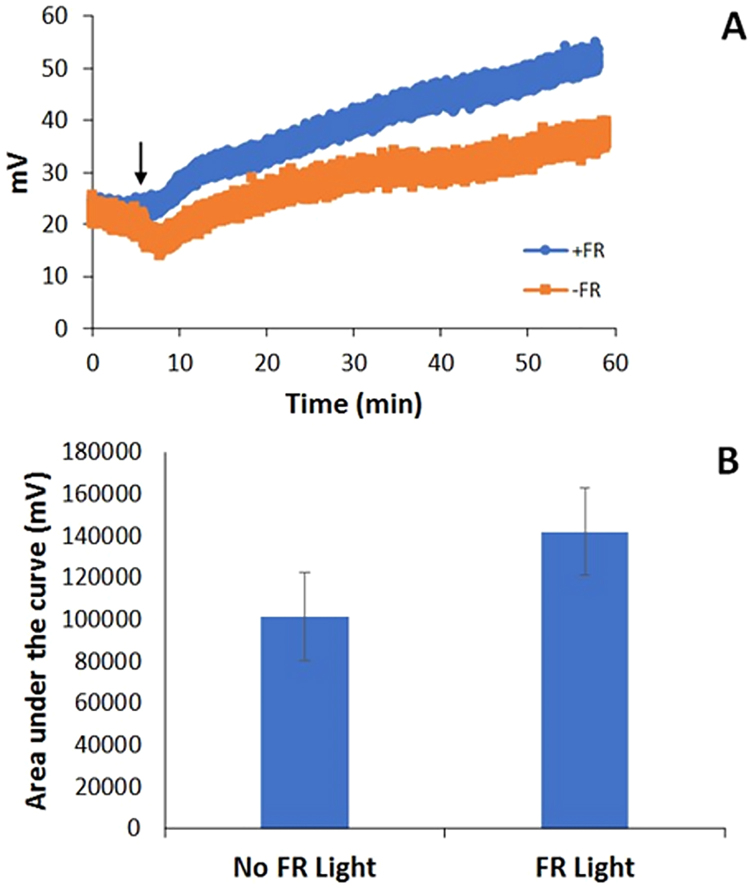
One method that has been used to assess RBC-dependent production of NO by nitrite is to directly measure NO using a chemiluminescence-based detector when RBCs are injected into a buffer containing nitrite [18], [24], [28], [61]. Using this method, we assessed whether exposure to far red light affected NO production from RBCs and nitrite. When RBCs were injected into nitrite-containing buffer, more NO was made with illumination than without illumination (Fig. 4). The far red light did not significantly increase the temperature of the samples in these experiments (n = 5, P = 0.64 with light vs without light, data not shown), thus ruling out heating as a mechanism. These data are consistent with the notion that nitrite bioactivation by RBCs, particularly through NO production, is enhanced in the presence of far red light.
Fig. 4.
Nitric oxide production by nitrite and red blood cells. Deoxygenated RBCs (50% Hct, 100 µL) were injected into 20 ml of PBS (pH 6.8) containing 20 mM nitrite in a three neck round bottom flask that was connected directly to a chemiluminescence-based nitric oxide analyzer with and without illumination by far red light (660 nm, 65 mW). Data are plotted as mean ± one standard error. (A) Representative raw data from the nitric oxide analyzer. RBCs were injected at 5 min and the signal increases more with light than without (indicated by arrow). (B) Average areas of the signals showing greater NO production in the presence of light (P = 0.04, n = 8).
4.4. Far red light and nitrite prolongs lag time of clotting in presence of RBCs
To further explore potential applications of combined nitrite/far red light treatment, we examined clotting in platelet poor plasma. Although the main effects of NO on clotting are known to be through its action as an anti-platelet agent, a few reports have shown effects on thrombin and fibrinogen [62], [63]. We did not observe any significant effects on the clot lysis time or maximum optical density (data not shown), but we did observe effects of nitrite and light on clotting lag time (time to reach 5% increase in OD, after initiating clotting with thrombin). In the absence of RBCs, nitrite had no effect on the lag time of clotting (P = 0.78, Fig. 5). When deoxygenated RBCs and nitrite were incubated with the clotting mixtures and the RBCs spun out prior to clotting, nitrite treatment was found to prolong the lag time compared to when nitrite was absent (Fig. 5). In addition, combination of nitrite and far red light treatment also prolonged the lag time compared to when these treatments were absent. Light treatment had no effect on lag time compared to no-light treatment in the absence of nitrite (P = 0.65, n = 8, data not shown). However, nitrite combined with light treatment resulted in longer lag times compared to light treatment alone (Fig. 5). To rule out a mechanism of light potentiation whereby the heating enhances the nitrite/Hb reaction we measured lag times for nitrite treated samples at 21 °C (the measured temperature of samples without light exposure) and 25 °C (the measured temperature of samples after 10 min of light exposure). We observed no differences at these temperatures (P = 0.26, n = 5, data not shown) Thus, our data support the notion that nitrite is bioactivated by RBCs and this action is potentiated by far red light.
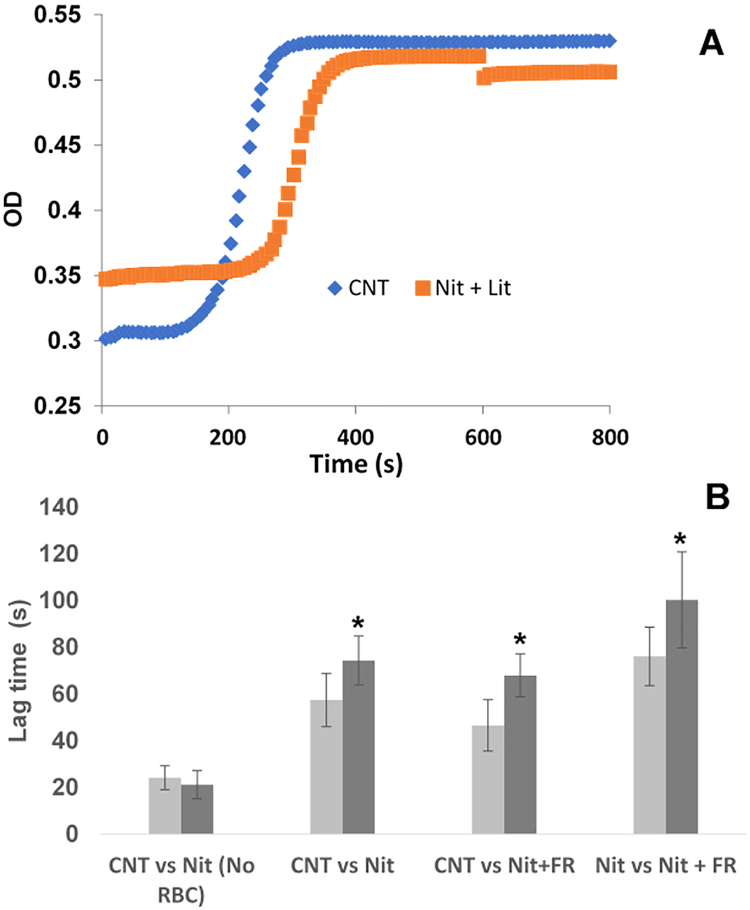
Fig. 5.
Effects of nitrite and far red light on clotting. Platelet poor plasma containing fibrinogen and an activation solution containing thrombin were prepared separately and, in some cases, incubated with deoxygenated RBCs (20% Hct), nitrite (10 μM), and/or treated with far red light (660 nm, 10 min, 107 mW). All experiments were performed pair-wise; that is two types of samples were performed side by side per day and the results compared. Data are plotted as mean ± one standard error. (A) Representative plots of optical density (OD) vs time after initiation of clotting, shown here for the case of a control (CNT, RBCs but no other treatment) vs a sample administered nitrite and light treatment (Nit + Lit). Except for the nitrite and light treatment, both samples were treated identically. (B) Average lag times and standard errors for repeated measures. In the absence of RBCs, nitrite had no effect on the lag time (control (CNT) vs Nit, P = 0.78, n = 5). When mixtures were pre-incubated with RBCs, nitrite treatment resulted in prolonged lag times compared to no nitrite treatment (CNT vs NIT, *P < 0.01, n = 8). Illumination with far red light (660 nm, 10 min) along with the nitrite treatment during pre-incubation with deoxygenated RBCs also prolonged lag time compared to when the nitrite and light treatments were both absent (CNT vs Nit+ FR, *P < 0.01, n = 5). Inclusion of the illumination treatment along with the nitrite treatment prolonged lag times compared to nitrite treatment alone (Nit vs Nit +FR, *P < 0.05, n = 10).
5. Discussion
We have demonstrated that far red light potentiates the effects of nitrite in a RBC-dependent manner under various physiological conditions in vitro and in vivo. We showed that far red light increases tissue oxygenation in hypoxia as does nitrite treatment and the combined treatment is additive in vivo in a rat model. In addition, far red light was shown to dramatically enhance RBC-mediated inhibition of platelet activation by nitrite. We presented substantial evidence supporting the involvement of NO signaling to account for these actions. In addition, we demonstrated additional potential for therapeutic application in thrombotic disease whereby nitrite and far red light increased the lag time for fibrin polymerization during clotting. These data support the hypothesis that RBCs carry NO activity that is enhanced by nitrite administration and exposure to far-red light that may be harnessed in therapeutic applications.
In 1968, Ehrreich and Furchgott showed that visible and ultraviolet light potentiates relaxation of isolated smooth muscle preparations [64]. However, the ultraviolet and blue light employed does not have very good transmittance in animal tissue. Infrared and far red light have better penetration and have been shown to increase tissue perfusion in several in vivo settings including in humans [51], [65], [66]. Nitrite has also been shown to increase tissue perfusion in vivo [67], [68]. Here, we show that nitrite treatment results in increased tissue oxygenation in rats and that far red light increases tissue oxygenation in rats under hypoxic conditions (breathing low FiO2) (Fig. 1). The combination of the two treatments was additive. The increased tissue oxygenation was likely due to increased perfusion, consistent with measured vessel diameters. The nitrite treatment employed here was extremely mild as evidenced by lack of significant elevations in plasma nitrite and nitrate. The trend in nitrate was stronger (P = 0.07) which is explained by the fact that the lifetime of plasma nitrite is only a few minutes while that of nitrate is several hours. The fact that the nitrite treatment was so mild could explain why nitrite treatment alone did not universally increase tissue oxygenation at all FiO2 compared to no nitrite treatment (Fig. 1). However, nitrite treatment did have a significant effect overall. These results suggest that more effective nitrite treatment, including dietary nitrate featuring beetroot juice which results in about 5-fold increases in plasma nitrite and nitrate in humans [69], could be even more effective in increasing tissue oxygenation under hypoxic conditions.
We showed that RBC-mediated inhibition of platelet activation by nitrite is dramatically potentiated by exposure to far red light (Fig. 2B). We also observed that light alone decreased platelet activation in the presence of RBCs (Fig. 2B) and hypothesize that this is due to endogenous NO modified RBCs. Similar to what we and others have shown for the case where light treatment is absent [23], [24], the presence of RBCs is required for inhibition of platelet activation due to light, nitrite, or their combination (Figs. 2 and 3B). We have previously shown that nitrite bioactivation by RBCs (in the absence of light) involves membrane surface thiols; when RBCs are treated with the cell-impermeable thiol-blocking agent DTNB, nitrite bioactivation is abrogated [25]. In examining vessel dilation ex vivo due to far red light exposure, Lohr et al. found that the effects of the light were dependent on an endothelium associated compound which they suggested to be either a nitrosothiol or a dinitrosyl iron complex (DNIC) [55]. Here, we have observed that DTNB abrogates the light/nitrite effects on RBC-mediated platelet inhibition (Fig. 3B). The fact that surface thiols are required for the effects argues against heating as a mechanistic component. Moreover, we conducted several control experiments where we measured nitrite effects at well-controlled temperatures using the temperatures of our samples before and after illumination. In all cases, we found that temperature (in the absence of light) had no effect on effects of nitrite bioactivation. Thus, our data supports the hypothesis (Fig. 6) that nitrite reacts with deoxygenated Hb which leads to an NO congener that forms a photolabile bond with a RBC surface thiol. This hypothesis is supported by increased production of NO detected by chemiluminescence when a RBC/nitrite solution is exposed to far red light (Fig. 4). In addition to a nitrosothiol or a DNIC, this NO congener may be a heme-nitrosyl species that results from export of the nitrosylated heme from Hb as previously suggested [70], [71]. These NO congeners would be capable of avoiding NO dioxygenation that blocks NO signaling (Eq. (2)).
Fig. 6.
Potential mechanism of RBC-mediated nitrite bioactivation and its potentiation by far red light. Nitrite reacts with deoxygenated Hb to make NO which then binds other vacant hemes or forms a nitrosothiol (RSNO). The nitrosyl-heme or nitrosothiol is exported and binds a surface thiol. Another potential NO species that may form is a DNIC (not shown). The NO congener can then be transported in plasma and interact with platelets and other blood components and this action is potentiated by photolysis using far red light.
One weakness of our data that does not fully support the hypothesis of a NO-photolable RBC-surface thiol bond comes out of our ANOVA analysis of our tissue oxygenation and platelet activation data. If our hypothesis is correct, one would expect a synergistic effect of light and nitrite. Otherwise, one might think that light and nitrite are acting by two separate, but additive mechanisms. Except in the case where an outlier in the platelet data was excluded, ANOVA analysis did not show significant interaction between treatments, arguing against synergisim. However, other data from our study, including the fact that light enhanced NO production from nitrite and RBCs and synergistically increased lag time of clotting, support our hypothesis.
Throughout our studies here, we have employed different light intensities for different experiments depending on the optical set-up in the application. Our work could be strengthened by a more thorough dose response in terms of both nitrite and light intensity. Such a future study could better define potential clinical applications.
Illumination of RBCs in PRP with far red light led to inhibition of platelet activation, even without nitrite treatment (Fig. 2B). Since nitrite is always present in plasma, we suggest that the effects seen in Fig. 2B without in vitro nitrite treatment are due to endogenous RBC/nitrite reactions prior to blood draw resulting in RBCs that have NO modified surface thiols. The extent of these “NO”-bound RBCs that are present would depend on individuals’ plasma nitrite that can be greatly affected by diet and other factors [72], [73]. It would be interesting to see if dietary or other interventions aimed at increasing plasma nitrite would result in increased activity of RBCs in terms of their ability to provide NO activity upon far red illumination.
Several studies have demonstrated antiplatelet effects of nitrite including one performed with human volunteers [74]. However, the effects were modest. Motivated by the dramatic enhancement by far red light on RBC-mediated inhibition of platelet activation by nitrite, we explored whether nitrite and far red light treatment might have other potential benefits related to treating thrombosis. Potential of NO to treat thrombosis is primarily through its action in reducing platelet activation [5], [75], [76], [77], [78], however, recent work suggests that it will also affect other aspects of clotting [62]. Previously, it was found that adding NO to thrombin and/or fibrinogen decreases the rate of fibrin fiber formation with consequentially lower fibrin fiber density and forming thicker fibers [62]. In this work, we found that incubation of fibrinogen and thrombin with deoxygenated RBCs and nitrite results in a prolonged lag time in clotting measured using turbidity from fibrin polymerization (Fig. 5). In addition, illumination with far red light combined with nitrite treatment further increased the lag time compared to nitrite treatment alone. These data support the notion that far red light enhances nitrite bioactivation by RBCs, producing NO activity that may be useful in treating thrombosis and other pathological conditions.
We have shown that far red light potentiates nitrite bioactivation by RBCs, producing NO activity. One advantage of a combined nitrite/far red light treatment in therapeutics would be the ability to localize release of NO activity. Nitrite bioactivation itself is targeted to areas of hypoxia [14], [15] which would be beneficial in localized NO release for treating several conditions [79], [80], [81], [82]. Given its penetration depth (on the order of a few millimeters to about one centimeter [83]) far red light would provide additional targeted NO release for applications near the surface of the skin (such as in wound healing or some thrombotic events). In addition, one can imagine devices where far red light is applied internally through implantable devices (e.g. stents, IVC filters) so that one achieves local reduction in thrombosis. In our work, we used a range of light intensities (from 65 to 560 mW) and saw effects even with these low intensities. Further work is necessary to further elucidate the mechanism of erythrocytic bioactivation of nitrite and its potentiation by far-red light and to establish potential in therapeutics.
Acknowledgments
This work was supported by NIH grants HL058091, HL098032, GM099807, and funding from the Translational Science Center, Center for Molecular Signaling, and Center for Redox Biology Medicine at Wake Forest University. We acknowledge services provided by the Flow Cytometry Core Laboratory of the Comprehensive Cancer Center, supported in part by NCI, National Institutes of Health Grant P30 CA121291-37.
Acknowledgments
Disclosures and conflicts of interest
Drs. Kim-Shapiro and Gladwin are listed as a co-inventor on a patent related to use of nitrite in cardiovascular conditions.
Contributor Information
Nadeem Wajih, Email: wajihn@wfu.edu.
Swati Basu, Email: basus@wfu.edu.
Kamil B. Ucer, Email: ucerkb@wfu.edu.
Fernando Rigal, Email: rigafj17@wfu.edu.
Aryatara Shakya, Email: aryatara.shakya@salem.edu.
Elaheh Rahbar, Email: alipe17@wfu.edu.
Vidula Vachharajani, Email: Vidula.Vachharajani@wakehealth.edu.
Martin Guthold, Email: gutholdm@wfu.edu.
Mark T. Gladwin, Email: gladwinmt@upmc.edu.
Lane M. Smith, Email: lmsmith@wakehealth.edu.
Daniel B. Kim-Shapiro, Email: shapiro@wfu.edu.
References
- 1.Ignarro L.J., Byrns R.E., Buga G.M., Wood K.S. Endothelium-derived relaxing factor from pulmonary-artery and vein possesses pharmacological and chemical-properties identical to those of nitric-oxide radical. Circ. Res. 1987;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 2.Palmer R.M.J., Ferrige A.G., Moncada S. Nitric-oxide release accounts for the biological-activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.Furchgott R.F. Studies on relaxation of rabbit aorta by sodium nitrite: the basis for the proposal that the acid- activatable factor from bovine retractor penis is inorganic nitrite and the endothelium-derived relaxing factor is nitric oxide. In: Vanhoutte P.M., editor. Vasodilatation: Vascular Smooth Muscle, Peptides, Autonomic Nerves, and Endothelium. Raven Press; New York: 1988. pp. 401–414. [Google Scholar]
- 4.Ignarro L.J., Byrns R.E., Wood K.S. Biochemical and pharmacological properties of endothelium-derived relaxing factor and its similarity to nitric oxide radical. In: Vanhoutte P.M., editor. Vasodilatation: Vascular Smooth Muscle, Peptides, Autonomic Nerves, and Endothelium. Raven Press; New York: 1988. pp. 427–435. [Google Scholar]
- 5.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88(8):756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 6.Radomski M.W., Palmer R.M.J., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric-oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer A., Wiesmann F., Neubauer S., Eigenthaler M., Bauersachs J., Channon K. Rapid regulation of platelet activation in vivo by nitric oxide. Circulation. 2004;109:1819–1822. doi: 10.1161/01.CIR.0000126837.88743.DD. [DOI] [PubMed] [Google Scholar]
- 8.Radomski M.W., Palmer R.M.J., Moncada S. The role of nitric-oxide and CGMP in platelet-adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 9.Roberts W., Michno A., Aburima A., Naseem K.M. Nitric oxide inhibits von Willebrand factor-mediated platelet adhesion and spreading through regulation of integrin alpha(IIb)beta(3) and myosin light chain. J. Thromb. Haemost. 2009;7(12):2106–2115. doi: 10.1111/j.1538-7836.2009.03619.x. [DOI] [PubMed] [Google Scholar]
- 10.Adams M.R., Jessup W., Hailstones D., Celermajer D.S. L-arginine reduces human monocyte adhesion to vascular endothelium and endothelial expression of cell adhesion molecules. Circulation. 1997;95(3):662–668. doi: 10.1161/01.cir.95.3.662. [DOI] [PubMed] [Google Scholar]
- 11.Jin H.-B., Yang Y.-B., Song Y.-L., Zhang Y.-c., Li Y.-R. Lipoic acid attenuates the expression of adhesion molecules by increasing endothelial nitric-oxide synthase activity. Mol. Biol. Rep. 2013;40(1):377–382. doi: 10.1007/s11033-012-2071-4. [DOI] [PubMed] [Google Scholar]
- 12.Kubes P., Suzuki M., Granger D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 1991;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer T., Preik M., Rassaf T., Strauer B.E., Deussen A., Feelisch M., Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA. 2001;98(22):12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X.L., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon R.O., Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 15.Helms C.C., Liu X., Kim-Shapiro D.B. Recent insights into nitrite signaling processes in blood. Biol. Chem. 2017;398(3):319–329. doi: 10.1515/hsz-2016-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Brooks, The action of nitrite on Haemoglobin in the absence of oxygen, in: Proceedings Royal Soc. London - Series B, Biol. Sci. 123, 1937, pp. 368–382.
- 17.Doyle M.P., Pickering R.A., Deweert T.M., Hoekstra J.W., Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256(23):12393–12398. [PubMed] [Google Scholar]
- 18.Huang Z., Shiva S., Kim-Shapiro D.B., Patel R.P., Ringwood L.A., Irby C.E., Huang K.T., Ho C., Hogg N., Schechter A.N., Gladwin M.T. Enzymatic function of hemoglobin as a nitrite reductase that produces Nitric oxide under allosteric control. J. Clin. Investig. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle M.P., Hoekstra J.W. Oxidation of nitrogen-oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 1981;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 20.Eich R.F., Li T.S., Lemon D.D., Doherty D.H., Curry S.R., Aitken J.F., Mathews A.J., Johnson K.A., Smith R.D., Phillips G.N., Olson J.S. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35(22):6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 21.Herold S., Exner M., Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40(11):3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 22.Crawford J.H., Isbell T.S., Huang Z., Shiva S., Chacko B.K., Schechter A.N., Darley-Usmar V.M., Kerby J.D., Lang J.D., Jr, Kraus D., Ho C., Gladwin M.T., Patel R.P. Hypoxia, red blood cells and nitrite regulate NO-dependent hypoxic vasodilatation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srihirun S., Sriwantana T., Unchern S., Kittikool D., Noulsri E., Pattanapanyasat K., Fucharoen S., Piknova B., Schechter A.N., Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wajih N., Basu S., Jailwala A., Kim H.W., Ostrowski D., Perlegas A., Bolden C.A., Buechler N.L., Gladwin M.T., Caudell D.L., Rahbar E., Alexander-Miller M.A., Vachharajani V., Kim-Shapiro D.B. Potential therapeutic action of nitrite in sickle cell disease. Redox Biol. 2017;12:1026–1039. doi: 10.1016/j.redox.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wajih N., Liu X., Shetty P., Basu S., Wu H., Hogg N., Patel R.P., Furdui C.M., Kim-Shapiro D.B. The role of red blood cell S-nitrosation in nitrite bioactivation and its modulation by leucine and glucose. Redox Biol. 2016;8:415–421. doi: 10.1016/j.redox.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu S., Grubina R., Huang J., Conradie J., Huang Z., Jeffers A., Jiang A., He X., Azarov I., Seibert R., Mehta A., Patel R., King S.B., Hogg N., Ghosh A., Gladwin M.T., Kim-Shapiro D.B. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 2007;3(12):785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 27.Nagababu E., Ramasamy S., Abernethy D.R., Rifkind J.M. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;278(47):46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 28.Webb A.J., Milsom A.B., Rathod K.S., Chu W.L., Qureshi S., Lovell M.J., Lecomte F.M.J., Perrett D., Raimondo C., Khoshbin E., Ahmed Z., Uppal R., Benjamin N., Hobbs A.J., Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008;103(9) doi: 10.1161/CIRCRESAHA.108.175810. 957–U114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche C.J., Cassera M.B., Dantsker D., Hirsch R.E., Friedman J.M. Generating S-nitrosothiols from hemoglobin mechanisms, conformational dependence, and physiological relevance. J. Biol. Chem. 2013;288(31):22408–22425. doi: 10.1074/jbc.M113.482679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelo M., Singel D.J., Stamler J.S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. USA. 2006;103(22):8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Hemann C., Abdelghany T.M., El-Mahdy M.A., Zweier J.L. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J. Biol. Chem. 2012;287(43):36623–36633. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiva S., Huang Z., Grubina R., Sun J.H., Ringwood L.A., MacArthur P.H., Xu X.L., Murphy E., Darley-Usmar V.M., Gladwin M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 33.Rassaf T., Flogel U., Drexhage C., Hendgen-Cotta U., Kelm M., Schrader J. Nitrite reductase function of deoxymyoglobin - oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007;100(12):1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 34.Li H.T., Samouilov A., Liu X.P., Zweier J.L. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J. Biol. Chem. 2004;279(17):16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 35.Millar T.M., Stevens C.R., Benjamin N., Eisenthal R., Harrison R., Blake D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427(2):225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 36.Gautier C., van Faassen E., Mikula I., Martasek P., Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem. Biophys. Res. Commun. 2006;341(3):816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Aamand R., Dalsgaard T., Jensen F.B., Simonsen U., Roepstorff A., Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2009;297(6):H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 38.Li H.T., Kundu T.K., Zweier J.L. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J. Biol. Chem. 2009;284(49):33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H., Cui H., Kundu T.K., Alzawahra W., Zweier J.L. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyide oxidase. J. Biol. Chem. 2008;283(26):17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y.R., Chen L.C., Liu X., Li H.T., Zweier J.L., Mason M.P. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the Hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radic. Biol. Med. 2004;37:1591–1603. doi: 10.1016/j.freeradbiomed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Basu S., Azarova N.A., Font M.D., King S.B., Hogg N., Gladwin M.T., Shiva S., Kim-Shapiro D.B. Nitrite reductase activity of cytochrome c. J. Biol. Chem. 2008;283(47):32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiso M., Tejero J., Basu S., Azarov I., Wang X., Simplaceanu V., Frizzell S., Jayaraman T., Geary L., Shapiro C., Ho C., Shiva S., Kim-Shapiro D.B., Gladwin M.T. Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 2011;286(20):18277–18289. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Keceli G., Cao R., Su J.T., Mi Z.Y. Molybdenum-containing nitrite reductases: spectroscopic characterization and redox mechanism. Redox Rep. 2017;22(1):17–25. doi: 10.1080/13510002.2016.1206175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Krizowski S., Fischer-Schrader K., Niks D., Tejero J., Sparacino-Watkins C., Wang L., Ragireddy V., Frizzell S., Kelley E.E., Zhang Y.Z., Basu P., Hille R., Schwarz G., Gladwin M.T. Sulfite oxidase catalyzes single-electron transfer at molybdenum domain to reduce nitrite to nitric oxide. Antioxid. Redox Signall. 2015;23(4):283–294. doi: 10.1089/ars.2013.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C., Wajih N., Liu X., Basu S., Janes J., Marvel M., Keggi C., Helms C.C., Lee A.N., Belanger A.M., Diz D.I., Laurienti P.J., Caudell D.L., Wang J., Gladwin M.T., Kim-Shapiro D.B. Mechanisms of human erythrocytic bioactivation of nitrite. J. Biol. Chem. 2015;290(2):1281–1294. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desmet K.D., Paz D.A., Corry J.J., Eells J.T., Wong-Riley M.T.T., Henry M.M., Buchmann E.V., Connelly M.P., Dovi J.V., Liang H.L., Henshel D.S., Yeager R.L., Millsap D.S., Lim J., Gould L.J., Das R., Jett M., Hodgson B.D., Margolis D., Whelan H.T. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed. Laser Surg. 2006;24(2):121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y.Y., Sharma S.K., Carroll J., Hamblin M.R. Biphasic dose response in low level light therapy - an update. Dose-Response. 2011;9(4):602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uozumi Y., Nawashiro H., Sato S., Kawauchi S., Shima K., Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010;42(6):566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 49.Eells J.T., Wong-Riley M.T.T., VerHoeve J., Henry M., Buchman E.V., Kane M.P., Gould L.J., Das R., Jett M., Hodgson B.D., Margolis D., Whelan H.T. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4(5–6):559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Blanco N.J., Maddox W.T., Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J. Neuropsychol. 2017;11(1):14–25. doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X.L., Tian F.H., Soni S.S., Gonzalez-Lima F., Liu H.L. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016;6 doi: 10.1038/srep30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karu T.I., Pyatibrat L.V., Kolyakov S.F., Afanasyeva N.I. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B-Biol. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Wong-Riley M.T.T., Liang H.L., Eells J.T., Chance B., Henry M.M., Buchmann E., Kane M., Whelan H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins - role of cytochrome c oxidase. J. Biol. Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 54.Lohr N.L., Keszler A., Pratt P., Bienengraber M., Warltier D.C., Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J. Mol. Cell. Cardiol. 2009;47(2):256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keszler A., Lindemer B., Weihrauch D., Jones D., Hogg N., Lohr N.L. Red/near infrared light stimulates release of an endothelium dependent vasodilator and rescues vascular dysfunction in a diabetes model. Free Radic. Biol. Med. 2017;113(Suppl. C):157–164. doi: 10.1016/j.freeradbiomed.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keszler A., Lindemer B., Hogg N., Weihrauch D., Lohr N.L. Wavelength-dependence of vasodilation and NO release from S-nitrosothiols and dinitrosyl iron complexes by far red/near infrared light. Arch. Biochem. Biophys. 2018;649:47–52. doi: 10.1016/j.abb.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Batenburg W.W., Kappers M.H.W., Eikmann M.J., Ramzan S.N.A., de Vries R., Danser A.H.J. Light-induced vs. bradykinin-induced relaxation of coronary arteries: do S-nitrosothiols act as endothelium-derived hyperpolarizing factors? J. Hypertens. 2009;27(8):1631–1640. doi: 10.1097/HJH.0b013e32832bff54. [DOI] [PubMed] [Google Scholar]
- 58.Plass C.A., Loew H.G., Podesser B.K., Prusa A.M. Light-induced vasodilation of coronary arteries and its possible clinical implication. Ann. Thorac. Surg. 2012;93(4):1181–1187. doi: 10.1016/j.athoracsur.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 59.Smith L.M., Varagic J., Yamaleyeva L.M. Photoacoustic imaging for the detection of hypoxia in the rat femoral artery and skeletal muscle microcirculation. Shock. 2016;46(5):527–530. doi: 10.1097/SHK.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pieters M., Philippou H., Undas A., de Lange Z., Rijken D.C., Mutch N.J. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J. Thromb. Haemost. 2018;16(5):1007–1012. doi: 10.1111/jth.14002. [DOI] [PubMed] [Google Scholar]
- 61.Webb A., Bond R., McLean P., Uppal R., Benjamin N., Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA. 2004;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helms C.C., Kapadia S., Gilmore A.C., Lu Z.X., Basu S., Kim-Shapiro D.B. Exposure of fibrinogen and thrombin to nitric oxide donor ProliNONOate affects fibrin clot properties. Blood Coagul. Fibrinolysis. 2017;28(5):356–364. doi: 10.1097/MBC.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponczek M.B., Nowak P., Wachowicz B. The effects of nitronium ion on nitration, carbonylation and coagulation of human fibrinogen. Gen. Physiol. Biophys. 2008;27(1):55–58. [PubMed] [Google Scholar]
- 64.Ehrreich S.J., Furchgott R.F. Relaxation of mammalian smooth muscles by visible and ultraviolet radiation. Nature. 1968;218(5142):682. doi: 10.1038/218682a0. [DOI] [PubMed] [Google Scholar]
- 65.Wang X.L., Tian F.H., Reddy D.D., Nalawade S.S., Barrett D.W., Gonzalez-Lima F., Liu H.L. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: a broadband near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 2017;37(12):3789–3802. doi: 10.1177/0271678X17691783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian F.H., Hase S.N., Gonzalez-Lima F., Liu H.L. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg. Med. 2016;48(4):343–349. doi: 10.1002/lsm.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cabrales P. Low dose nitrite enhances perfusion after fluid resuscitation from hemorrhagic shock. Resuscitation. 2009;80(12):1431–1436. doi: 10.1016/j.resuscitation.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kenjale A.A., Ham K.L., Stabler T., Robbins J.L., Johnson J.L., VanBruggen M., Privette G., Yim E., Kraus W.E., Allen J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Presley T.D., Morgan A.R., Bechtold E., Clodfelter W., Dove R.W., Jennings J.M., Kraft R.A., King S.B., Laurienti P.J., Rejeski W.J., Burdette J.H., Kim-Shapiro D.B., Miller G.D. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24(1):34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ignarro L.J., Adams J.B., Horwitz P.M., Wood K.S. Activation of soluble guanylate-cyclase by no-hemoproteins involves No-heme exchange - comparison of heme-containing and heme-deficient enzyme forms. J. Biol. Chem. 1986;261(11):4997–5002. [PubMed] [Google Scholar]
- 71.Kleschyov A.L. The NO-heme signaling hypothesis. Free Radic. Biol. Med. 2017;112:544–552. doi: 10.1016/j.freeradbiomed.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 72.Gladwin M.T., Schechter A.N., Kim-Shapiro D.B., Patel R.P., Hogg N., Shiva S., Richard I., Cannon O., Kelm M., Wink D.A., Espey M.G., Oldfield E.H., Pluta R.M., Freeman B.A., Jack J., Lancaster R., Feelisch M., Lundberg J.O. The emerging biology of the nitrite anion. Nat. Chem. Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 73.Lundberg J.O., Gladwin M.T., Ahluwalia A., Benjamin N., Bryan N.S., Butler A., Cabrales P., Fago A., Feelisch M., Ford P.C., Freeman B.A., Frenneaux M., Friedman J., Kelm M., Kevil C.G., Kim-Shapiro D.B., Kozlov A.V., Lancaster J.R., Lefer D.J., McColl K., McCurry K., Patel R.P., Petersson J., Rassaf T., Reutov V.P., Richter-Addo G.B., Schechter A., Shiva S., Tsuchiya K., van Faassen E.E., Webb A.J., Zuckerbraun B.S., Zweier J.L., Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5(12):865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Velmurugan S., Kapil V., Ghosh S.M., Davies S., McKnight A., Aboud Z., Khambata R.S., Webb A.J., Poole A., Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radomski M.W., Rees D.D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br. J. Pharmacol. 1992;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simon D.I., Stamler J.S., Jaraki O., Keaney J.F., Osborne J.A., Francis S.A., Singel D.J., Loscalzo J. Antiplatelet properties of protein S-nitrosothiols derived from nitric-oxide and endothelium-derived relaxing factor. Arterioscler. Thromb. 1993;13(6):791–799. doi: 10.1161/01.atv.13.6.791. [DOI] [PubMed] [Google Scholar]
- 77.Vilahur G., Baldellou M.I., Segales E., Salas E., Badimon L. Inhibition of thrombosis by a novel platelet selective S-nitrosothiol compound without hemodynamic side effects. Cardiovasc. Res. 2004;61(4):806–816. doi: 10.1016/j.cardiores.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 78.Vilahur G., Badimon L. Nitric oxide donors as platelet inhibitors. In: Freedman J.E., Loscalzo J., editors. New Therapeutic Agents in Thrombosis and Thrombolysis. Informa Healthcare; New York: 2009. pp. 499–516. [Google Scholar]
- 79.Gutsaeva D.R., Montero-Huerta P., Parkerson J.B., Yerigenahally S.D., Ikuta T., Head C.A. Molecular mechanisms underlying synergistic adhesion of sickle red blood cells by hypoxia and low nitric oxide bioavailability. Blood. 2014;123(12):1917–1926. doi: 10.1182/blood-2013-06-510180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marsch E., Sluimer J.C., Daemen M. Hypoxia in atherosclerosis and inflammation. Curr. Opin. Lipidol. 2013;24(5):393–400. doi: 10.1097/MOL.0b013e32836484a4. [DOI] [PubMed] [Google Scholar]
- 81.Bovill E.G., van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu. Rev. Physiol. 2011;73(73):527–545. doi: 10.1146/annurev-physiol-012110-142305. [DOI] [PubMed] [Google Scholar]
- 82.Bento C.F., Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54(8):1946–1956. doi: 10.1007/s00125-011-2191-8. [DOI] [PubMed] [Google Scholar]
- 83.Ash C., Dubec M., Donne K., Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017;32(8):1909–1918. doi: 10.1007/s10103-017-2317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]