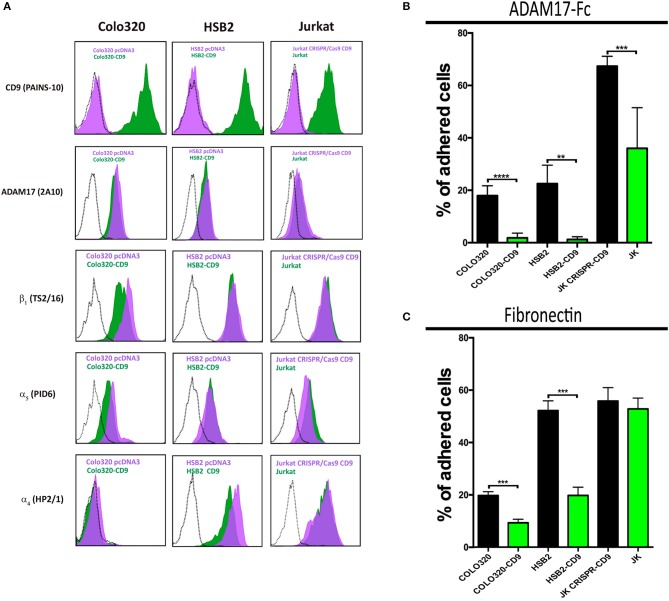
Figure 3.
Ectopic expression or knocking-out of CD9 regulates α5β1-mediated cell adhesion to ADAM17-Fc and Fn. Flow cytometric detection of cell surface CD9 (mAb PAINS-10), ADAM17 (mAb 2A10), β1 (mAb TS2/16), α5 (mAb P1D6), and α4 (mAb HP2/1) on Colo320, HSB2 and Jurkat cells (A). The black dotted line histograms correspond to negative controls, green filled histograms correspond to the expression of the indicated molecules on CD9 expressing cells (Colo320-CD9, HSB2-CD9, Jurkat) and purple filled histograms correspond to the expression of the indicated molecules on their respective CD9-lacking counterparts (Colo320, HSB2, Jurkat-CRISPR-CD9). The percentages of adhesion of paired variants of Colo320, HSB2 and Jurkat cells, either expressing CD9 (Colo320-CD9, HSB2-CD9, Jurkat; green bars) or lacking expression of this tetraspanin (Colo320, HSB2, Jurkat CRISPR-CD9; black bars) on plastic-immobilized ADAM17-Fc (B) or on fibronectin (C). In all cases, cells were stimulated with PMA (200 ng/ml) for 2 h, loaded with the fluorescent probe BCECF-AM and then allowed to adhere to plastic-immobilized ligands ADAM17-Fc (20 μg/ml) or Fn (7.5 μg/ml) for 60 min at 37°C under conditions of integrin activation in the presence of Mn2+ (200 μM). Data are presented as the percentage of adhered cells (means ± SEM of three experiments each performed in triplicates). Statistical analysis was carried out using the two-tailed paired T-test. **, *** and **** denote p < 0.01, p < 0.001 and p < 0.0001, respectively.