Figure 7.
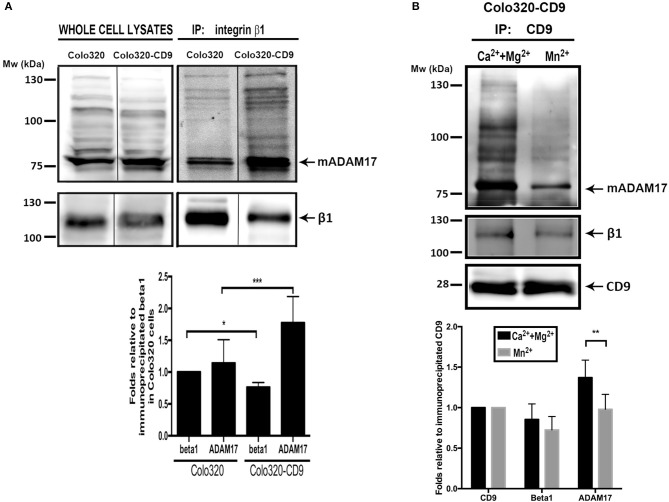
Cell surface ADAM-17 is more efficiently co-immunoprecipitated with integrin α5β1 from Colo320-CD9 cells than from Colo320 cells. (A) Only cell surface integrin α5β1 molecules were selectively immunoprecipitated by incubating the cells with mAb Lia1/2 (anti-β1) in the presence of Ca2++Mg2+ (500 μM each) and washing the excess non-bound antibody prior to cell lysis and immunoprecipitation. Immunoprecipitated integrin α5β1 and co-immunoprecipitated ADAM17 were detected by immunoblotting with the anti-β1 (TS2/16) and anti ADAM17 (A300D) mAbs, respectively. The gel shown is representative of five different experiments. The graph below shows the densitometric quantitation of the amount of precipitated integrin and co-immunoprecipitated ADAM17 (means ± SEM) from five different experiments, normalized to β1 precipitated in Colo320 cells in each experiment. (B) Integrin α5β1 and ADAM17 are coimmunoprecipitated with CD9 from cell surface TEMs. CD9 was immunoprecipitated with mAb PAINS10 as described for integrin β1 in (A) but under two different extracellular cation conditions: in the presence of Ca2++Mg2+ (500 μM each) or Mn2+ (200 μM). Immunoprecipitated CD9 and co-immunoprecipitated β1 and ADAM17 were detected by immunoblotting with mAbs PAINS10 (anti-CD9), TS2/16 (anti-β1), and A300D (anti ADAM17), respectively. The gel shown is representative of four different experiments. The graph below represents the densitometric quantitation of the amount of precipitated CD9 and co-immunoprecipitated integrin β1 and ADAM17 (means ± SEM) normalized to the immunoprecipitated CD9 in each of the four independent experiments. Statistical analysis was carried out using two-tailed paired T-test. *p < 0.05, **p < 0.01, ***p < 0.001.