Figure 8.
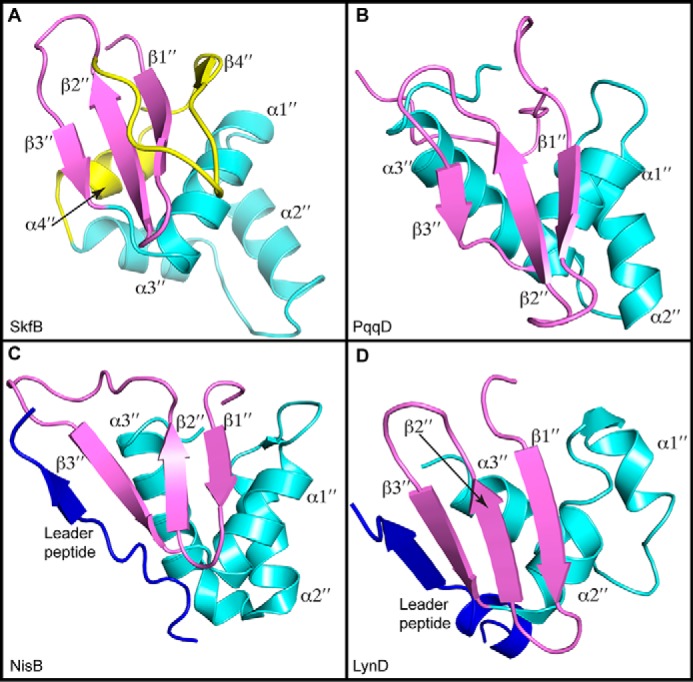
Structural comparisons of RRE domains. A, SkfB exhibits a canonical RRE comprising a three-strand antiparallel β-sheet (β1″–β3″ in purple) and three consecutive α-helices (α1″–α3″ in cyan). Following the RRE motif, SkfB folds into an additional α-helix, α4″, and β-strand, β4″ (yellow). B, the small peptide-binding protein PqqD (PDB code 5SXY) is a standalone RRE domain. C, the RRE domain of NisB (PDB code 4WD9) binds the leader peptide sequence of the peptide substrate NisA (blue) by extending the antiparallel β-sheet or the wing. D, the leader peptide of PatE (blue) also binds to the RRE domain of LynD (PDB code 4V1T) through interactions with the wing.
