Figure 9.
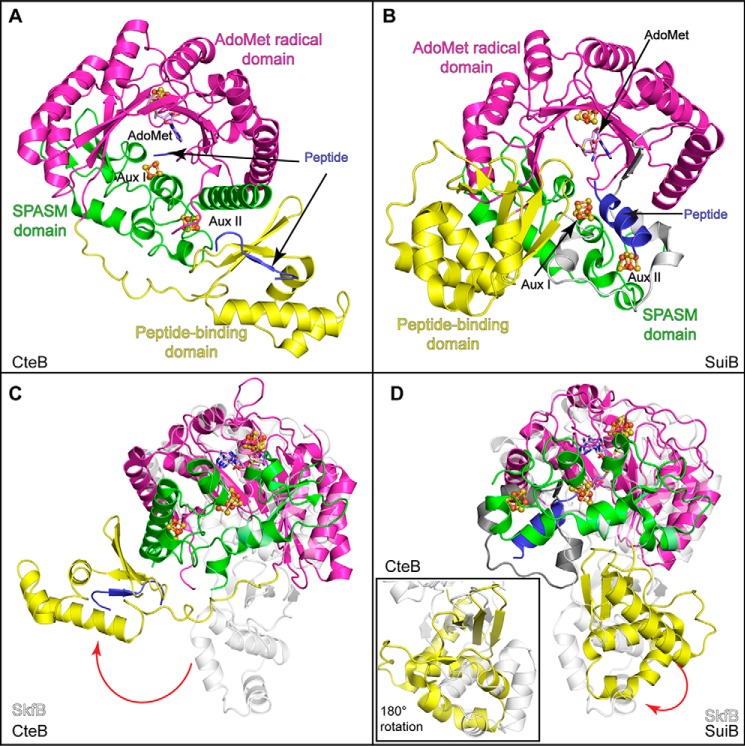
Structural comparisons of SkfB with other AdoMet radical enzymes involved in RIPP biosynthesis. A, CteB (PDB code 5WGG) exhibits a trimodular fold composed of a peptide-binding or RRE domain (yellow) followed by an AdoMet-binding domain (magenta), which binds the AdoMet radical cluster and AdoMet (lilac). The C-terminal end of CteB binds two clusters, Aux I and Aux II, using the SPASM domain architecture (green). The leader sequence of CteA (blue) binds to the RRE domain by extending the antiparallel β-sheet. B, SuiB (PDB code 5V1T) demonstrates a similar modular fold to CteB (A). Interestingly, the substrate for SuiB, SuiA (blue), is observed making contacts with the insertion (gray) between the AdoMet radical domain (magenta) and the SPASM domain (green) and not with the peptide-binding domain (yellow). C, the AdoMet radical and Twitch domains of SkfB (white) overlay well with CteB, but the peptide-binding domains are located on opposite sides of the AdoMet radical domain. D, SkfB (white) overlays well with SuiB, and the positions of the N-terminal domains show modest differences. A view of the overlaid N-terminal domains SkfB and SuiB, rotated 180°, is shown in the inset.