Figure 7.
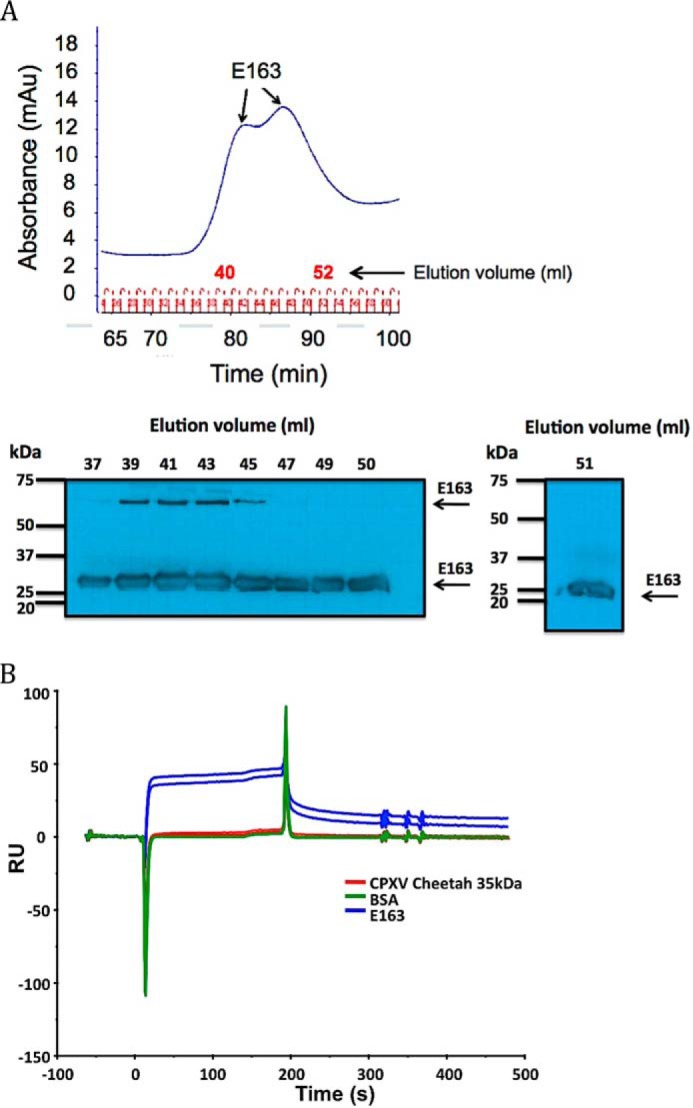
Oligomeric state of purified E163 protein. A, analysis of purified E163 protein by size exclusion chromatography. Recombinant E163 expressed in the baculovirus system and purified with Ni2+-nitrilotriacetic acid columns was analyzed by gel filtration chromatography. The arrows indicate the two peaks in the chromatogram and the elution volume of the fractions is indicated in red. The lower panel shows Western blot analysis with anti-E163 specific antibodies of selected fractions containing E163. The elution volume of the fractions analyzed by Western blot, molecular size in kDa, and arrows marking the position of the E163 protein are indicated. B, homotypic interaction of E163 in SPR-binding assays. The E163 protein was immobilized onto a BIAcore chip and the interaction with 2 different concentrations (250 and 500 nm) of E163 (blue), cowpox virus 35-kDa strain Cheetah (red), and BSA (green) was tested. The flow rate was 10 μl/min and the association (3 min) and the dissociation phases were monitored. A sensorgram is shown.
