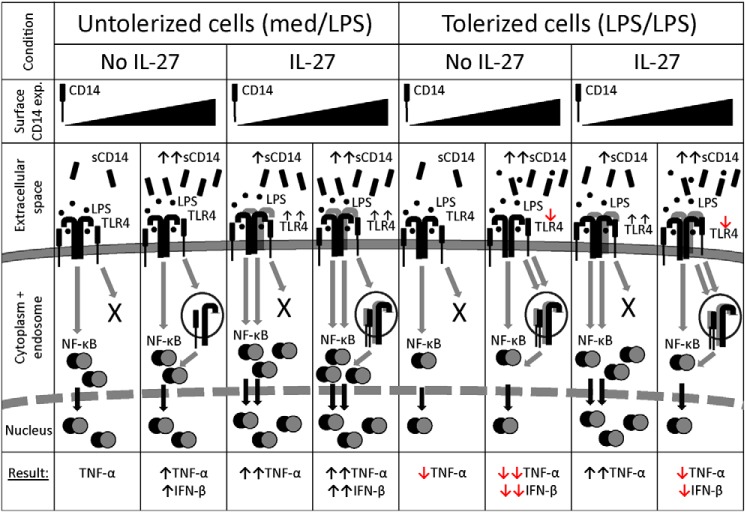
Figure 9.
A balance between membrane and soluble CD14 modulates the effects of IL-27 on endotoxin tolerance. In untolerized CD14low-expressing cells, LPS-TLR4-CD14 ligation induces NF-κB nuclear translocation and TNF-α production. However, these cells do not have sufficient CD14 to induce TLR4 endocytosis required for IFN-β production. In untolerized CD14high-expressing cells, LPS stimulation results in NF-κB nuclear localization, TNF-α production, and CD14-mediated TLR4 endocytosis, resulting in IFN-β production via endosomal TLR4 signaling. In CD14low-expressing cells, IL-27 enhances sCD14 and TLR4 expression to enhance LPS-TLR4 ligation, resulting in greater NF-κB nuclear localization and TNF-α production. IL-27–treated CD14high-expressing cells also exhibit enhanced TLR4 expression for more TNF-α production. High levels of surface CD14 allow for TLR4 endocytosis and IFN-β production in response to LPS, even though IL-27 does not affect sCD14 in these cells. Endotoxin-tolerized CD14low-expressing cells exhibit reduced NF-κB nuclear translocation, correlating with less TNF-α production compared with untolerized cells. In tolerized CD14high-expressing cells, TLR4 internalization and less sCD14 reduces subsequent responsiveness to LPS, resulting in less TNF-α and IFN-β production. In CD14low-expressing cells, LPS + IL-27 treatment enhances TLR4 expression, resulting in greater LPS signaling and NF-κB nuclear translocation for even greater TNF-α production than untolerized cells. CD14high-expressing cells exposed to LPS + IL-27 internalize TLR4, and because of the excess sCD14, the 2° LPS ligation is inhibited. Together, this results in decreased TNF-α and IFN-β production relative to untolerized cells. However, IL-27–mediated TLR4 up-regulation accounts for increased cytokine production relative to tolerized cells.