Figure 11.
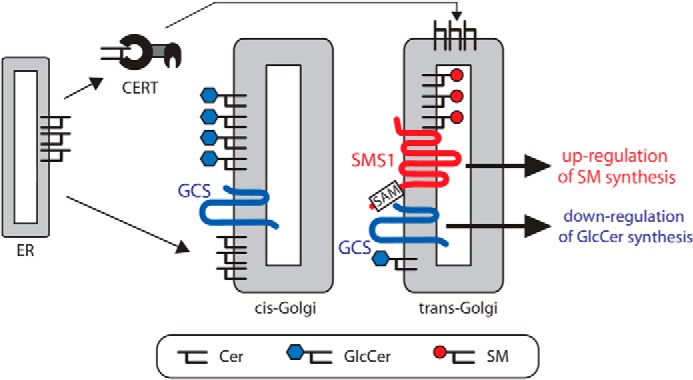
Heteromeric complex between SMS1 and GCS regulates Cer metabolism. A model for the role of the SMS1–GCS heteromeric complex in cellular Cer metabolisms is shown. The de novo synthesized Cer is transported from the ER to the trans-Golgi in a CERT-dependent manner or to the cis-Golgi in a CERT-independent manner. The Cer transported to cis-Golgi is mainly used for GlcCer synthesis, because SMS1 is localized mainly in the medial/trans-Golgi, whereas GCS has a broader distribution than SMS1 in the Golgi. We found that SMS1 forms a heteromeric complex with GCS through its N terminus, and the complex is involved in the regulation of the cellular Cer. The SAM domain in the N terminus of SMS1 is associated with the stability of the heteromeric complex. Based on these results, we propose a model in which SM synthesis is up-regulated by stable SMS1–GCS heterodimer formation, whereas GlcCer synthesis is down-regulated under conditions in which both proteins are in close proximity. Given that SMS1 and GCS partially co-localize in the cisternae, especially in medial/trans-Golgi, CERT-transported Cer in trans-Golgi might be partially regulated by the SMS1–GCS heteromeric complex.
