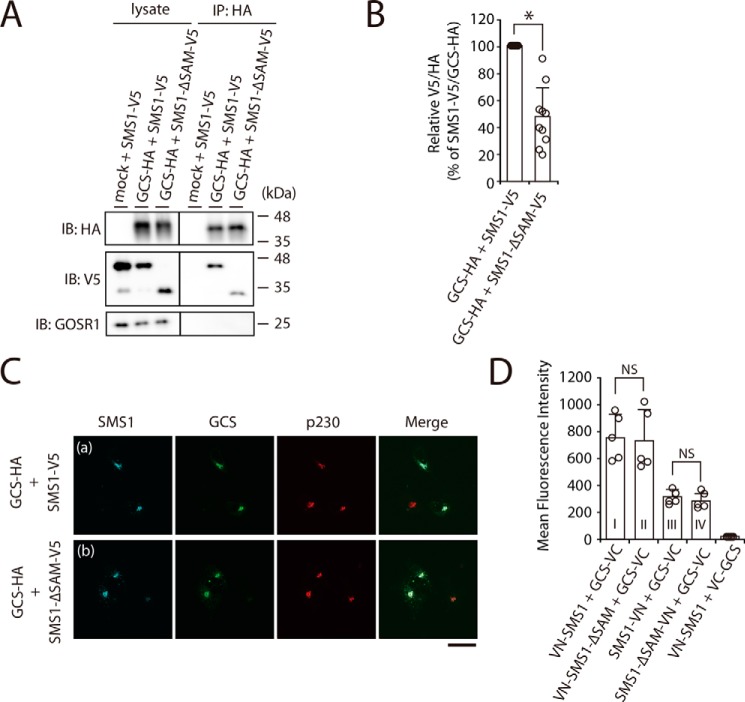
Figure 4.
The N-terminal SAM domain of SMS1 is important for the stable SMS1–GCS heteromeric complex. A and B, COS7 cells were transfected with the following combinations of plasmids: HA-tagged GCS or mock and V5-tagged SMS1 (WT) or V5-tagged SMS1–ΔSAM. At 24 h post-transfection, proteins from cell lysates were immunoprecipitated using an anti-HA affinity gel. A, cell lysates and precipitated proteins were analyzed by immunoblotting (IB) with anti-HA, anti-V5 antibody, or anti-GOSR1 antibody. Left panel, cell lysate; right panel, immunoprecipitation (IP). B, the quantities of V5-tagged SMS1 or SMS1–ΔSAM precipitated with HA-tagged GCS are represented as the ratio of the band intensities. The values represent the means ± S.D. from 10 independent experiments. *, p < 0.01. C, COS7 cells were co-transfected with indicated plasmids. At 24 h post-transfection, the cells were co-stained with anti-V5 and anti-HA antibodies, followed by appropriate Alexa Fluor–conjugated secondary antibodies and analyzed by confocal microscopy. Localization was confirmed by co-staining with antibody against the trans-Golgi network marker p230. Blue, SMS1 (WT or ΔSAM); green, GCS; red, p230. Scale bar, 50 μm. The results shown are from one experiment representative of three independent experiments. D, COS7 cells were co-transfected with indicated combination of plasmids. At 24 h post-transfection, the cells were subjected to immunostaining with antibodies, and Venus fluorescence complementation was quantified by flow cytometry. The values represent the means ± S.D. from five independent experiments. NS, not significant.