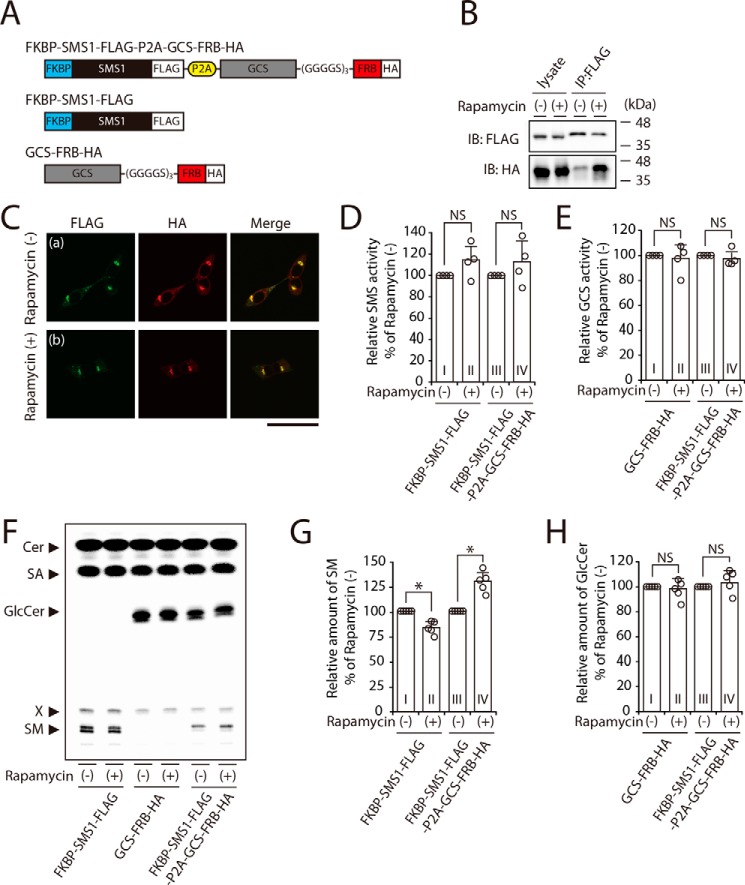
Figure 7.
SM synthesis is increased by rapamycin-induced heterodimerization with FKBP-fused SMS1 and FRB-fused GCS. A, schematic representation of the chimeric proteins engineered for rapamycin-induced heterodimerization. FKBP-fused SMS1 was linked with the FRB-fused GCS through a picorna virus-derived 2A autocleavage site (P2A). The locations of FLAG and HA tags are indicated. B and C, SMS1–GCS DKO cells were transfected with FKBP–SMS1–FLAG–P2A–GCS–FRB–HA. At 24 h post-transfection, the cells were treated with 500 nm rapamycin for 3 h. B, proteins from cell lysates were immunoprecipitated (IP) using an anti-FLAG affinity gel. Cell lysates and precipitated proteins were analyzed by immunoblotting (IB) with anti-FLAG or anti-HA antibody. The blots are from one experiment representative of three independent experiments. C, immunocytochemistry analysis; all cells were co-stained with anti-FLAG and anti-HA antibodies, followed by appropriate Alexa Fluor–conjugated secondary antibodies, and analyzed by confocal microscopy. Green, FKBP–SMS1–FLAG; red, GCS–FRB–HA. Scale bar, 50 μm. The images shown are from one experiment representative of three independent experiments. D-H, SMS1–GCS DKO cells were transfected with indicated plasmids. At 24 h post-transfection, each cells were treated with or without 500 nm rapamycin for 3 h. D and E, assay for SMS (D) and GCS (E) activities in vitro; all cell lysates were incubated with C6–NBD–Cer in the absence (D) or presence of UDP–Glc (E). The values are expressed as percentages of enzyme activity to rapamycin-untreated control cells and represent the means ± S.D. from four independent experiments. F–H, assay for SMS (F and G) and GCS (F and H) activities in vivo by metabolic labeling; all cells were cultured in the culture medium containing 0.5 μCi of [14C]stearic acid (SA) and 500 nm rapamycin for 3 h. Lipids were extracted, saponified, and separated by TLC (F). The values of SM (G) and GlcCer (H) are expressed as percentages of radioactive SM or GlcCer to rapamycin-untreated control cells and represent the means ± S.D. from five independent experiments. *, p < 0.01; NS, not significant.