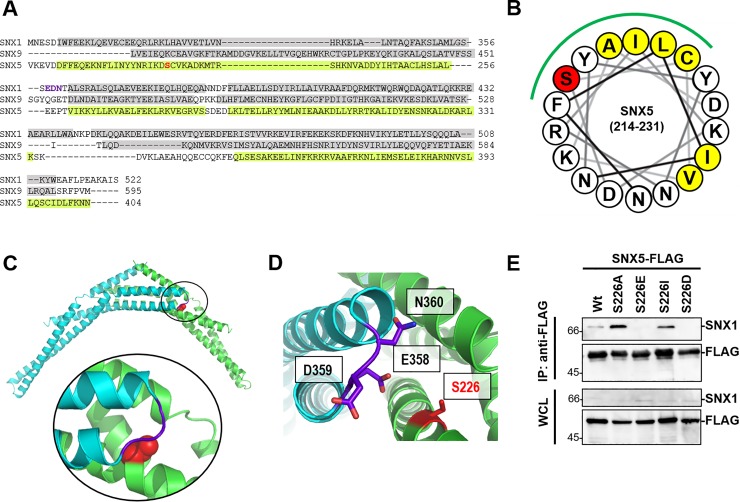
Fig 6. SNX5 S226 localize in the hydrophobic region of α-helix on BAR domain.
(A) Alignment of the BAR domain of three SNX family members. The α-helices (gray) of SNX1 and SNX9 are indicated based on the SNX1 crystal structure and the SNX9 structure. The α-helices of SNX5 (light green) are predicted by PSI-PRED. (B) Cartoon of the arrangement of the 18-residue around S226 (red). Green line shows hydrophobic region in which hydrophobic residue is assembled. (C) The model of the heterodimer of SNX1-BAR and SNX5-BAR. SNX1-BAR and SNX5-BAR are shown in cyan and green, respectively. S226 is shown as red space filling sphere. Around S226 is extended in circle. (D) Close-up view of S226 in the interface of the heterodimer of SNX1-BAR and SNX5-BAR. The charged residues in SNX1-BAR within 10Å of S226 and S226 are shown in stick format. Images (C) and (D) were produced using PyMOL softwere. (E) FLAG pull-down assays were performed on lysates of HEK293T cells expressing SNX5 Wt, S226A, S226E, S226I and S226D by lipofection. The FLAG fusion protein and SNX1 were detected by immunoblotting with anti-FLAG, anti-SNX1 antibodies.