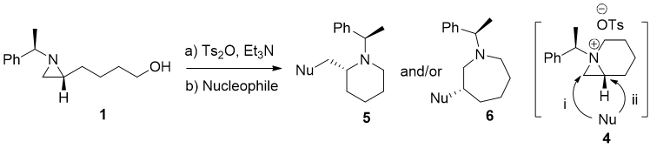
| ||||||
| Entry | Substrate | Nucleophile | Solventa,b | Time (h) | Ratio (5/6)c | Yieldd (%) |
| a | Aziridine (1) | NaN3 | CH3CN | 9 | 41/59 | 94 |
| b | Aziridine (1) | NaN3 | 1,4-dioxane | 2.0 | 40/60 | 84 |
| c | Aziridine (1) | CsF | CH3CN | 8.0 | 46/54 | 79 |
| d | Aziridine (1) | n-Bu4NBr | CH3CN | 12.0 | Complex | - |
| e | Aziridine (1) | NaI | CH3CN | 12.0 | Complex | - |
| f | Aziridine (1) | NaCN | CH3CN | 8.0 | 92/08 | 92 |
| g | Aziridine (1) | NaSCN | CH3CN | 11.0 | 91/09 | 93 |
| h | Aziridine (1) | NaOAc | CH3CN | 12.0 | 60/40 | 90 |
| i | Aziridine (1) | NaOMe | CH3CN | 11.0 | 58/42 | 93 |
| j | Aziridine (1) | NaOH | 1,4-dioxane | 2.0 | 15/85 | 93 |
| k | Aziridine (1) | BnNH2 | CH3CN | 14.0 | 35/65 | 87 |
| l | Aziridine (1) | Phenol | CH3CN | 15.0 | 47/53 | 56 |
| m | Aziridine (1) | NaOBz | CH3CN | 13.0 | 48/52 | 76 |
| n | Aziridine (1a) | NaN3 | CH3CN | 9.5 | 40/60 | 91e |
| [a]CH2Cl2 was used as solvents for the preparation of 2-(4-tosyloxybutyl)aziridine. [b]Tosylation was performed at 0 to 25 °C. | ||||||
| [c]Determined by 1H NMR. [d]Combined yield of 5 and 6. [e]Inseparable mixture of 5n and 6n from isomeric aziridine 1a. | ||||||
| [f] All reactions were performed at 25 °C except the entries b and j (110 °C). [g]Concentration is 0.1 M |

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.