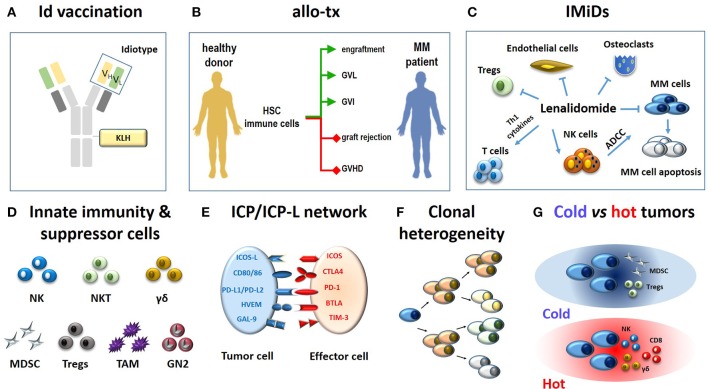
Figure 1.
Immune-based approaches in MM patients (A–C) and major hurdles to their definitive clinical success (D-G). (A) The monoclonal immunoglobulin produced by myeloma cells is a very specific TAA. The antigenic determinants localized in the complementary determining regions of monoclonal heavy and light chains (yellow and green rectangles) are termed idiotypes (Id) and are tumor-specific. Id specifities have been used to address tumor-specific immune responses. A vaccine formulation consisting of Id-specific proteins conjugated with KLH as immunogenic carrier has been shown to generate very specific and long-lasting anti-myeloma immune responses (6). (B) The ultimate goals of allogeneic transplantation (allo-tx) are to ensure a rapid blood engraftment mediated by donor HSC and concurrently address donor immune effector cells to eliminate residual malignant cells (GVL) and to control post-transplant infections (GVI; green lines). Ideally, these goals are achieved in the absence of graft rejection and/or GVHD (red lines) (72). So far, it has not been possible to clearly separate GVL from GVHD in the clinical practice. (C) Immunomodulatory drugs like lenalidomide fine-tune multiple immune functions in MM patients: (i) they enhance and potentiate the cytototoxic and ADCC activity of T cells and NK cells, respectively; (ii) they inhibit myeloma cell growth and induce apoptosis; (iii) they inhibit osteoclasts, ECs, and Tregs suppressor functions. (D) The role of innate effector cells such as NK cells, NKT cells and γδ T cells has been neglected when initial immunotherapy approaches have been developed; the need to overcome or neutralize the suppressor role of MDSCs, Tregs, TAMs, and suppressive neutrophils type II (GN2) was unkown and not addressed. (E) Another major hurdle is represented by the ICP/ICP-L immune suppressive circuitry. The interactions between ICP expressed by effector cells (ICOS, CTLA-4, PD-, BTLA, TIM-3) and ICP-L expressed by myeloma cells and bystander cells in the TME (ICOS-L, CD80/CD86, PDL-1/PDL-2, HVEM, GAL-9) impair anti-myeloma immunity. (F) MM is characterized by clonal and subclonal diversity which is shaped over time by repeated treatments, responses, and relapses. This clonal heterogeneity facilitates the immune escape of myeloma cells. (G) The TME immune infiltration discriminates between cold and hot tumors. The former are characterized by the local recruitment and/or activation of immune suppressor cells like Tregs and MDSC; the latter are characterized by the presence of cytotoxic cells (NK, CD8, γδ T cells). Clonal diversity, mutational load, and treatments are key factors to drive the immune infiltration of cold vs. hot tumors. Hot tumors are more sensitive to immunotherapy than cold tumors. The MM TME is closer to cold than hot tumors. Id, idiotype; KLH, keyhole limpet hemocyanin; allo-tx, allogeneic transplantation; HSC, hematopoietic stem cells; MM, multiple myeloma; graft-vs.-leukemia, GVL; graft-vs.-infections, GVI; graft-vs. host desease (GVHD); IMIDs, immunomodulatory drugs; Tregs, regulatory T cells; MDSC, myeloid derived suppressor cell; tumor-associated macrophages (TAM); GN, granulocyte neutrofils; NK, natural killer; ADCC, antibody-dependent-cellular-cytotoxicity; NKT, natural killer T cells; ICP, immune checkpoint; ICP-L, immune checkpoint ligands; TAA: tumor associated antigen.