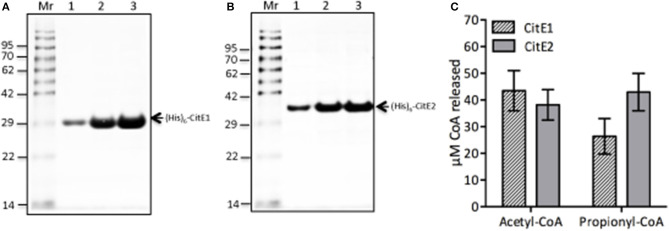
Figure 2.
(A,B) Expression and purification of (His)6-CitE1 and (His)6-CitE2. The recombinant proteins (His)6-CitE1 (A) and (His)6-CitE2 (B) were expressed and purified using Ni2+-NTA affinity chromatography. Loading pattern: (A) Un-Whole cell lysates of uninduced E. coli; In-Whole cell lysates of IPTG induced cells; Lanes (1–4)-Purified fractions of (His)6-CitE1. (B) Un-Whole cell lysates of uninduced E. coli; In-Whole cell lysates of IPTG induced E. coli; Lanes (1–5)- Purified fractions of (His)6-CitE2. (C) Biochemical characterization of (His)6-CitE1 and (His)6-CitE2. Both (His)6-CitE1 and (His)6-CitE2 exhibited acetyl-CoA lyase and propionyl-CoA lyase activities. The amount of CoA released in enzymatic reaction was quantified using DTNB reagent as described in Materials and Methods. The data shown in this panel is mean ± S.E. of CoA release obtained in enzymatic reactions from three independent experiments.