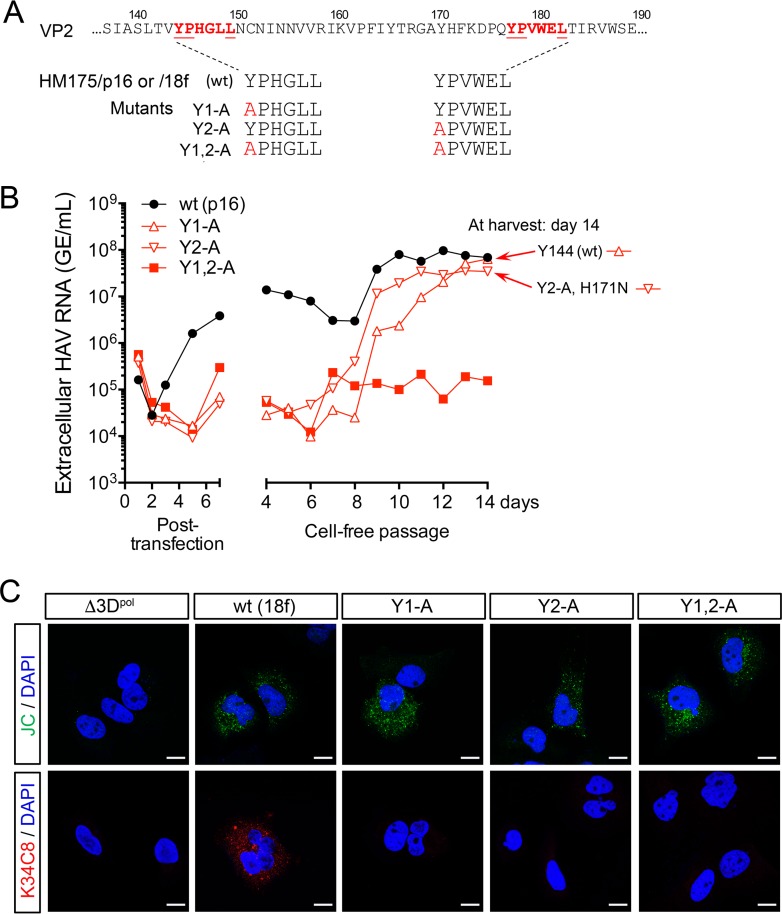
FIG 2.
Tyr-to-Ala mutations in the HAV VP2 YPX3L motifs ablate capsid assembly. (A) YPX3L late domain Tyr-to-Ala mutants constructed in the background of p16 and 18f virus (Fig. 1A). (B) Extracellular virus release following electroporation of Huh-7.5 cells with wt (HM175/p16) or related Y1-A, Y2-A, and Y1,2-A late domain mutants. Medium was replaced daily, and virus was quantified by reverse transcription-quantitative PCR (RT-qPCR). Cell-free virus was passaged from lysates harvested on day 7 postelectroporation, with supernatant fluid virus titers followed for an additional 14 days. Sequencing of viruses at day 14 postpassage revealed reversion of the Y1-A mutation to wt (Tyr144) and the presence of a second-site substitution in Y2-A (VP2 H171N). (C) Laser scanning confocal fluorescence microscopy of Huh-7.5 cells electroporated with the indicated wt (HM175/18f) or mutant RNA and fixed and stained 48 h later with either JC polyclonal human (top row) or K34C8 murine monoclonal anticapsid (bottom row) antibodies. The absence of K34C8 fluorescence despite abundant JC fluorescence in cells transfected with the mutant RNAs is consistent with the absence of capsid assembly. Nuclear counterstaining was with DAPI (blue). Δ3Dpol is a genome-length HAV RNA in which Gly-Ala-Ala has replaced the Gly-Asp-Asp motif in the 3Dpol RNA-dependent RNA polymerase. Bars, 10 µm.