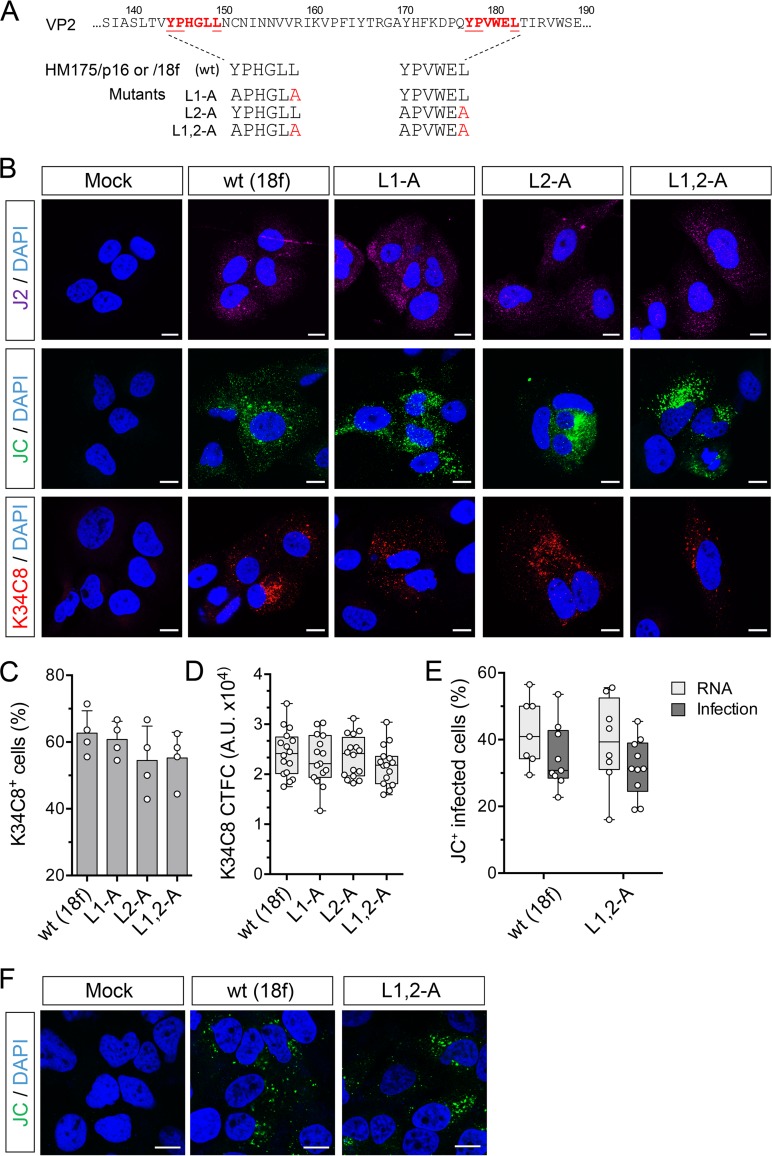
FIG 5.
Capsid assembly is not impaired by carboxy-terminal Leu-to-Ala substitutions within the VP2 YPX3L motifs of HAV. (A) YPX3L late domain Leu-to-Ala mutants constructed in the background of p16 and 18f viruses (Fig. 1A). (B) Confocal immunofluorescence microscopy of Huh-7.5 cells mock electroporated or electroporated with wt (HM175p16) or related L1-A, L2-A, or L1,2-A double mutant RNAs. Cells were fixed 48 h postelectroporation and stained with J2 (monoclonal anti-dsRNA), JC (polyclonal human anti-HAV), or K34C8 (monoclonal HAV anticapsid) antibodies. Strong K34C8 fluorescence in cells transfected with each of the mutants is indicative of efficient capsid assembly (22). Nuclear counterstaining was with DAPI (blue). Bars, 10 µm. (C) Percentage of cells stained with the K34C8 anticapsid monoclonal antibody following transfection with each of the mutants. Data shown are means ± standard deviations from 4 independent experiments. (D) Intensity of K34C8 fluorescence (CTFC, corrected total fluorescence intensity per cell) in cells transfected with wt or the indicated mutant viral RNA. All comparisons between cells transfected with mutant RNAs and those transfected with wt control RNAs were nonsignificant statistically (P > 0.29 by one-way ANOVA). A.U., arbitrary units. (E) Assay for infectious virus produced in cells transfected with wt (18f) or L1,2-A RNA. Results shown represent percent cells staining positively with JC antibody 48 h after RNA electroporation (RNA) or 48 h after inoculation of fresh cells with lysates of the electroporated cells (Infection). Data are from 7 to 10 low-power microscopy fields of cells under each condition and are representative of two independent experiments. (F) Confocal microscopic images of Huh-7.5 cells inoculated with cell-free lysates prepared from cells 48 h after electroporation with wt or L1,2-A RNA or no RNA (mock). Cells were stained with polyclonal JC antibody to HAV 48 h after inoculation.