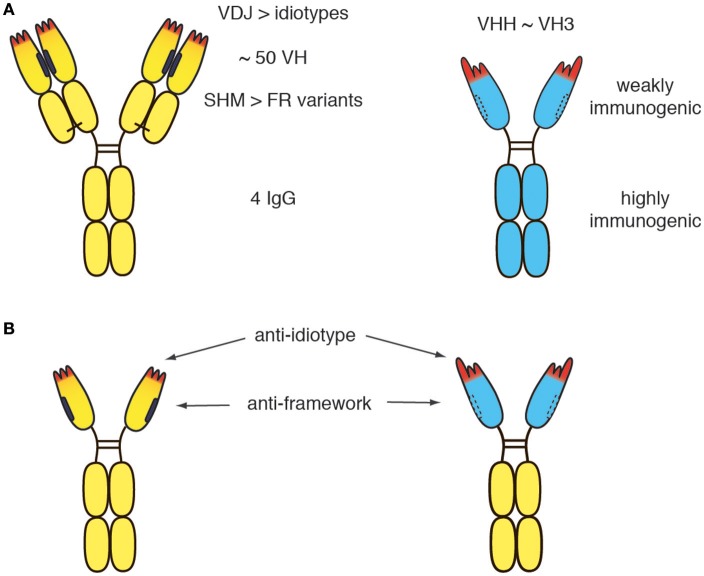
Figure 2.
Potential immunogenicity of heavy chain antibodies. (A) The human germline encodes ~50 distinct VH domains and 4 distinct IgG isotpyes. V-D-J recombination during B-cell development generates millions of distinct idiotypes (antigen binding paratopes, CDR regions 1, 2 indicated in red). Subsequent to antigen encounter, somatic hypermutation generates many more variant VH domains. During pregnancy, maternal IgGs are translocated through the placental trophoblasts to the fetus, leading to tolerization of the new born human immune system against millions of VH variants, but only 4 distinct IgG isotypes. (B) In germline configuration, llama VHH domains show ~80-90% amino acid sequence identitiy to human VH3 domains. A few amino acid substitutions in the VL face (mainly framework region 2, indicated by dashed lines) and a long CDR3 that can partially fold back onto this face largely account for the dramatically improved solubility of camelid VHH domains vs. human VH3 domains. The solubility of human VH can be improved by “camelization,” i.e., by replacing hydrophobic residues at the interface of the VL domain (indicated in black) with hydrophylic residues resembling those found in VHH domains. Conversely, camelid VHH domains can be “humanized,” i.e., by replacing amino acid residues in the framework with residues corresponding to germlin human VH domains. However, the idiotype of a therapeutic moAb or hcAb cannot be fully humanized without compromising binding to the target antigen. Similarly, the VL face cannot be fully humanized without compromising solubility. Therefore, small risks remain, that the patient will develop antibodies against the idiotype and/or against the (much smaller) hydrophilic VL face.