FIGURE 1.
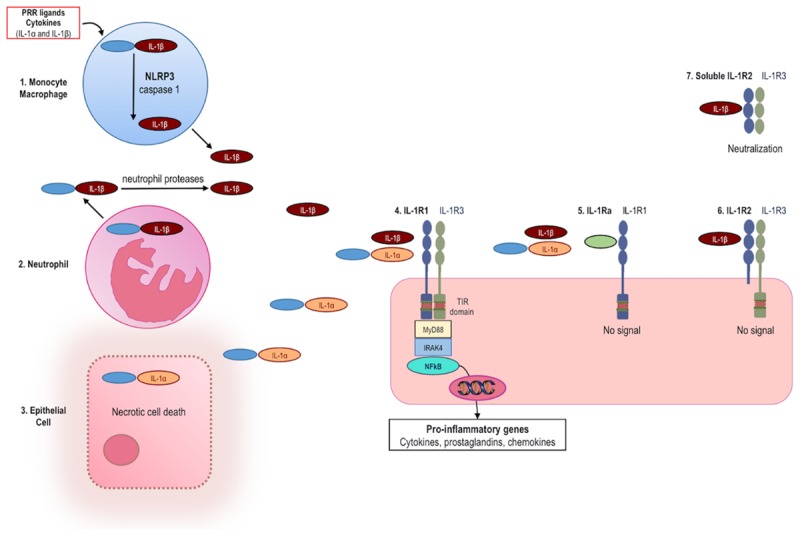
Production and release of IL-1, signaling and inhibition of IL-1 activities. (1) The IL-1β precursor is induced in monocytes/macrophages following engagement of pattern recognition receptors (PRR) or by pro-inflammatory cytokines, including IL-1α and IL-1β. IL-1β is synthesized as an inactive precursor (pro-IL-1β). Release of biologically active IL-1β takes place by enzymatic cleavage of the precursor protein by caspase-1. Activation of caspase-1 requires induction of the NLRP3 inflammasome. (2) Neutrophils release the IL-1β precursor into the extracellular space where it is cleaved to active IL-1β by neutrophil-derived proteases. (3) The IL-1α precursor is constitutively present in most epithelial cells and is fully active. Upon cell necrosis, the intracellular IL-1α precursor is released and acts as an alarmin. (4) Both IL-1α and IL-1β bind to IL-1 receptor type 1 (IL-1R1), which is followed by recruitment of the co-receptor IL-1R3 (formerly termed IL-1 receptor accessory protein, IL-1RAcP). The heterotrimer results in the approximation of the intracellular TIR domains of IL-1R1 and IL-1R3. MyD88, IL-1 receptor-associated kinase 4 (IRAK4), and NFκB are phosphorylated. NFκB induces transcription of pro-inflammatory genes. Mechanisms physiologically counteracting the activity of IL-1α and IL-1β include: (5) The IL-1 receptor antagonist (IL-1Ra, green) binds IL-1R1 and prevents binding of IL-1α and IL-1β, thereby resulting in no signal. (6) The IL-1 receptor type 2 (IL-1R2) preferentially binds IL-1SS, but lacking a cytoplasmic domain, acts as a decoy receptor and there is no signal. (7) Soluble IL-1R2 (extracellular domain only) binds IL-1β and forms a complex with soluble IL-1R3, resulting in neutralization of IL-1β.
