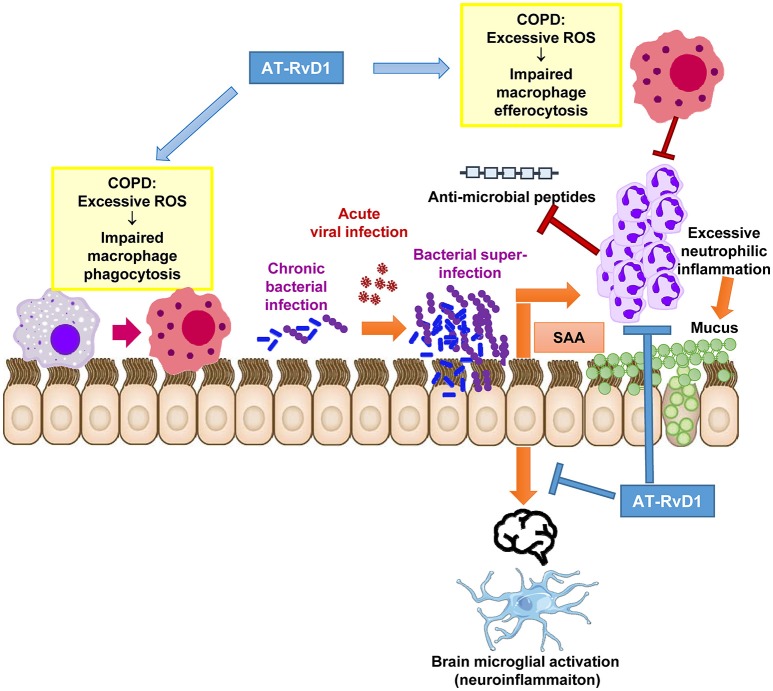
Figure 1.
Proposed therapeutic actions of AT-RvD1 during acute exacerbations in chronically infected COPD patients. Excessive inflammation and ROS are a characteristic feature of the COPD lung microenvironment. Excessive ROS directly impairs macrophage function preventing efficient phagocytosis of potentially pathogenic bacteria and efferocytosis (removal of dying neutrophils). This has deleterious effects on mucosal immunity and permits the establishment of chronic bacterial infection of the lower airways. Upon exposure to a newly acquired viral infection, which is a common trigger for AECOPD, the virus permits the further outgrowth of bacteria causing a bacterial super-infection in the lungs. SAA is significantly increased during co-infections and stimulates neutrophilic inflammation via FPR2/ALX-dependent mechanisms. Excessive neutrophilic inflammation can drive mucus hypersecretion and degrade anti-microbial peptides in the airways. We propose that the alternative FPR2/ALX agonist, AT-RvD1 can therapeutically intervene at critical pathological pathways that lead to bacterial super-infections. AT-RvD1 facilitates the resolution of inflammation during co-infections by improving the phagocytic clearance of bacteria and efferocytosis of apoptotic neutrophils in the lungs. It also potently suppresses neutrophil migration, thereby limiting tissue damage, mucus secretion and anti-microbial peptide degradation caused by ongoing inflammation. AT-RvD1 may also reduce neuroinflammation consequent to serious lung co-infections as brain microglia are activated by SAA in manner that is suppressed by AT-RvD1.